14.5 Uncommon Amino Acids
... • Amino acids are the primary structures that make up a chain of protein • Different sequences of peptide and protein molecules allows for the protein to carry out its functions • The formula for calculating the possible numbers of peptides and proteins for a chain of n amino acids by raising it to ...
... • Amino acids are the primary structures that make up a chain of protein • Different sequences of peptide and protein molecules allows for the protein to carry out its functions • The formula for calculating the possible numbers of peptides and proteins for a chain of n amino acids by raising it to ...
of a protein
... chains they differ in size, shape, charge, H-bonding capacity, hydrophobic character and chemical reactivity twenty amino acids build up all proteins in all species in the evolutionary tree (with few exceptions; this “alphabet” is several billion years old) ...
... chains they differ in size, shape, charge, H-bonding capacity, hydrophobic character and chemical reactivity twenty amino acids build up all proteins in all species in the evolutionary tree (with few exceptions; this “alphabet” is several billion years old) ...
Abstract
... hand motifs and by zinc- and copper-binding, to a site located at the dimer interface. These facts and our recent observation that S100 proteins have intrinsic β-aggregation propensity [3] have prompted us to investigate S100B self-assembly reactions and co-aggregation phenomena involving other abun ...
... hand motifs and by zinc- and copper-binding, to a site located at the dimer interface. These facts and our recent observation that S100 proteins have intrinsic β-aggregation propensity [3] have prompted us to investigate S100B self-assembly reactions and co-aggregation phenomena involving other abun ...
biomolecule ppt
... • They consist of a carboxyl group (COOH) and an amino group NH2 • Peptide bonds form between amino acids (polypeptide = many peptide bonds = protein!) ...
... • They consist of a carboxyl group (COOH) and an amino group NH2 • Peptide bonds form between amino acids (polypeptide = many peptide bonds = protein!) ...
HERE
... The tRNA attaches AMINO ACIDS together to FORM PROTEINS – This is called Protein Synthesis ...
... The tRNA attaches AMINO ACIDS together to FORM PROTEINS – This is called Protein Synthesis ...
Chemistry of Life - Haughton Science
... Present Carbon Hydrogen Oxygen ONLY ! There is no specific ratio. ...
... Present Carbon Hydrogen Oxygen ONLY ! There is no specific ratio. ...
Biological Building Blocks Andrew Rylaarsdam
... hoped. With these peptides synthesized and characterized, we are beginning to study how they bind to metals, and how this affects their folding. One property that is indicative of metals binding to a protein or peptide structure is how much light the molecules absorb. Some of the amino acid “buildin ...
... hoped. With these peptides synthesized and characterized, we are beginning to study how they bind to metals, and how this affects their folding. One property that is indicative of metals binding to a protein or peptide structure is how much light the molecules absorb. Some of the amino acid “buildin ...
Proteins
... to hydrogen bonds forming between amide and carboxyl groups. There are two possible types of secondary structure: ...
... to hydrogen bonds forming between amide and carboxyl groups. There are two possible types of secondary structure: ...
Introduction to Proteins
... • Examples of protein functions Alcohol dehydrogenase oxidizes alcohols to aldehydes or ketones ...
... • Examples of protein functions Alcohol dehydrogenase oxidizes alcohols to aldehydes or ketones ...
Proteins - Northwest ISD Moodle
... make up a protein. - the interactions of the R groups on each amino acid cause the molecule to bend and fold – different arrangements create different shapes - as a result- the order of amino acids determines the shape of the protein - shape determines function - changing a single amino acid can cha ...
... make up a protein. - the interactions of the R groups on each amino acid cause the molecule to bend and fold – different arrangements create different shapes - as a result- the order of amino acids determines the shape of the protein - shape determines function - changing a single amino acid can cha ...
Cell Transport Notes Learning Targets 8. Explain the significance of
... 10 Explain the terms: hypotonic, hypertonic or isotonic in relationship to the internal environments of cells. ...
... 10 Explain the terms: hypotonic, hypertonic or isotonic in relationship to the internal environments of cells. ...
File
... Amine (NH2) and carboxylic acid (COOH) groups attached to carbon Only thing different is side chain…R-group (side chain) ...
... Amine (NH2) and carboxylic acid (COOH) groups attached to carbon Only thing different is side chain…R-group (side chain) ...
Unit One: Introduction to Physiology: The Cell and General Physiology
... Protein Metabolism • Use of Proteins for Energy- once cells are filled to their limit with proteins, any additional aa are degraded and used for energy or stored as fat or glycogen ...
... Protein Metabolism • Use of Proteins for Energy- once cells are filled to their limit with proteins, any additional aa are degraded and used for energy or stored as fat or glycogen ...
Proteins - Mr Waring`s Biology Blog
... When more amino acids are added to a dipeptide, a polypeptide chain is formed. A protein consists of one or more polypeptide chains folded into a highly specific 3D shape. There are up to four levels of structure in a protein: primary, secondary, tertiary and quaternary. Each of these play an import ...
... When more amino acids are added to a dipeptide, a polypeptide chain is formed. A protein consists of one or more polypeptide chains folded into a highly specific 3D shape. There are up to four levels of structure in a protein: primary, secondary, tertiary and quaternary. Each of these play an import ...
Carbon Isomers
... Ribonucleic acid (RNA) • RNA similar to DNA except – Contains ribose instead of deoxyribose – Contains uracil instead of thymine ...
... Ribonucleic acid (RNA) • RNA similar to DNA except – Contains ribose instead of deoxyribose – Contains uracil instead of thymine ...
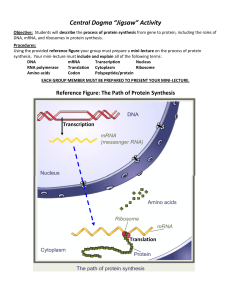
DNA to Proteins
... cytoplasm. It is fed through a ribosome The bases on the mRNA strand are matched by another type of RNA called transfer RNA or tRNA. • Every group of 3 bases on mRNA codes for 1 amino acid ...
... cytoplasm. It is fed through a ribosome The bases on the mRNA strand are matched by another type of RNA called transfer RNA or tRNA. • Every group of 3 bases on mRNA codes for 1 amino acid ...
Protein

Proteins (/ˈproʊˌtiːnz/ or /ˈproʊti.ɨnz/) are large biomolecules, or macromolecules, consisting of one or more long chains of amino acid residues. Proteins perform a vast array of functions within living organisms, including catalyzing metabolic reactions, DNA replication, responding to stimuli, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific three-dimensional structure that determines its activity.A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than about 20-30 residues, are rarely considered to be proteins and are commonly called peptides, or sometimes oligopeptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residues in a protein is defined by the sequence of a gene, which is encoded in the genetic code. In general, the genetic code specifies 20 standard amino acids; however, in certain organisms the genetic code can include selenocysteine and—in certain archaea—pyrrolysine. Shortly after or even during synthesis, the residues in a protein are often chemically modified by posttranslational modification, which alters the physical and chemical properties, folding, stability, activity, and ultimately, the function of the proteins. Sometimes proteins have non-peptide groups attached, which can be called prosthetic groups or cofactors. Proteins can also work together to achieve a particular function, and they often associate to form stable protein complexes.Once formed, proteins only exist for a certain period of time and are then degraded and recycled by the cell's machinery through the process of protein turnover. A protein's lifespan is measured in terms of its half-life and covers a wide range. They can exist for minutes or years with an average lifespan of 1–2 days in mammalian cells. Abnormal and or misfolded proteins are degraded more rapidly either due to being targeted for destruction or due to being unstable.Like other biological macromolecules such as polysaccharides and nucleic acids, proteins are essential parts of organisms and participate in virtually every process within cells. Many proteins are enzymes that catalyze biochemical reactions and are vital to metabolism. Proteins also have structural or mechanical functions, such as actin and myosin in muscle and the proteins in the cytoskeleton, which form a system of scaffolding that maintains cell shape. Other proteins are important in cell signaling, immune responses, cell adhesion, and the cell cycle. Proteins are also necessary in animals' diets, since animals cannot synthesize all the amino acids they need and must obtain essential amino acids from food. Through the process of digestion, animals break down ingested protein into free amino acids that are then used in metabolism.Proteins may be purified from other cellular components using a variety of techniques such as ultracentrifugation, precipitation, electrophoresis, and chromatography; the advent of genetic engineering has made possible a number of methods to facilitate purification. Methods commonly used to study protein structure and function include immunohistochemistry, site-directed mutagenesis, X-ray crystallography, nuclear magnetic resonance and mass spectrometry.