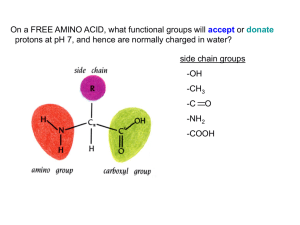
Biochemistry
... • All living things are made up of four classes of large biological molecules: carbohydrates, lipids, proteins, and nucleic acids • Within cells, small organic molecules are joined together to form larger molecules • Macromolecules are large molecules composed of thousands of covalently ...
... • All living things are made up of four classes of large biological molecules: carbohydrates, lipids, proteins, and nucleic acids • Within cells, small organic molecules are joined together to form larger molecules • Macromolecules are large molecules composed of thousands of covalently ...
Bio-Macromolecules Worksheet
... of the fatty acids is replaced by a phosphate group. This makes the phospholipid molecule polar at one end and nonpolar at the other. Phospholipids have a hydrophilic (polar) head - the phosphate and two hydrophobic (nonpolar) tails – the fatty acids. In water they orient themselves in a bilayer wit ...
... of the fatty acids is replaced by a phosphate group. This makes the phospholipid molecule polar at one end and nonpolar at the other. Phospholipids have a hydrophilic (polar) head - the phosphate and two hydrophobic (nonpolar) tails – the fatty acids. In water they orient themselves in a bilayer wit ...
Transition Elements/Coordination Chemistry
... Transition metals have electron configurations that fill the d orbitals last, yet when losing electrons the valence s electrons are generally lost first. When the atom zinc loses two electrons to become Zn+2 it is the 4s2 electrons that are lost… ...
... Transition metals have electron configurations that fill the d orbitals last, yet when losing electrons the valence s electrons are generally lost first. When the atom zinc loses two electrons to become Zn+2 it is the 4s2 electrons that are lost… ...
Homework One - Calderglen High School
... Explain why the first ionisation energy of potassium is less than the first ionisation energy of lithium. Potassium electron further away from nucleus and more shielding from inner ...
... Explain why the first ionisation energy of potassium is less than the first ionisation energy of lithium. Potassium electron further away from nucleus and more shielding from inner ...
LS1a Fall 2014 Lab 2 (PyMOL- Protein) question sheet Q1) (10 points)
... residues away? Hydrogen atoms bound to the backbone nitrogens of the peptide bonds act as the H-bond donors (the entire N-H group can also be considered the donor). Carbonyl oxygen atoms of the backbone peptide bonds act as the acceptors. The H-bond donating residue is 4 residues away from the H-bon ...
... residues away? Hydrogen atoms bound to the backbone nitrogens of the peptide bonds act as the H-bond donors (the entire N-H group can also be considered the donor). Carbonyl oxygen atoms of the backbone peptide bonds act as the acceptors. The H-bond donating residue is 4 residues away from the H-bon ...
Lab 1 activity, AMINO ACIDS - Cal State LA
... Proline has a nitrogen in an aliphatic ring system • Proline (Pro, P) - has a three carbon side chain bonded to the a-amino nitrogen • The heterocyclic pyrrolidine ring restricts the geometry of polypeptides ...
... Proline has a nitrogen in an aliphatic ring system • Proline (Pro, P) - has a three carbon side chain bonded to the a-amino nitrogen • The heterocyclic pyrrolidine ring restricts the geometry of polypeptides ...
Option B IB Chemistry Definitions HL
... Occurs in inner membrane of mitochondria, which contains different proteins and enzymes, incl. cytochromes. The H+ ions from the NADH2 (product from the citric acid cycle) move along cytochromes by repeated redox reactions, due to presence of stronger oxidizing agents. Enzyme cytochrome oxidase caus ...
... Occurs in inner membrane of mitochondria, which contains different proteins and enzymes, incl. cytochromes. The H+ ions from the NADH2 (product from the citric acid cycle) move along cytochromes by repeated redox reactions, due to presence of stronger oxidizing agents. Enzyme cytochrome oxidase caus ...
Chemical Reactions Practice Test
... show the number of g rams of each substance that react are the mo;lar masses of the substances that react show the valence electrons for each atom ...
... show the number of g rams of each substance that react are the mo;lar masses of the substances that react show the valence electrons for each atom ...
AP2A Ch2 Chemistry-2017
... (c) Chemical work Energy liberated during oxidation of food fuels used to regenerate ATP ...
... (c) Chemical work Energy liberated during oxidation of food fuels used to regenerate ATP ...
97KB - NZQA
... form and it would get warm. This reaction is a decomposition reaction, as a single reactant (hydrogen peroxide) forms two products (water and oxygen). Heat a small amount of each white solid in a boiling-tube. The boiling tube should have a bung in it, with a delivery tube going into a test-tube of ...
... form and it would get warm. This reaction is a decomposition reaction, as a single reactant (hydrogen peroxide) forms two products (water and oxygen). Heat a small amount of each white solid in a boiling-tube. The boiling tube should have a bung in it, with a delivery tube going into a test-tube of ...
Synthesis of new nitric oxide donor derivatives
... Properties of Ligands The ligands may be anions or neutral molecules . Neutral atoms arenot found in coordination agents. There are one factor that all ligands have in common that one non – bonded pairs of es , which is used to form a coordination covalent bond with metal. ...
... Properties of Ligands The ligands may be anions or neutral molecules . Neutral atoms arenot found in coordination agents. There are one factor that all ligands have in common that one non – bonded pairs of es , which is used to form a coordination covalent bond with metal. ...
3.1.2 Group 2
... Group 2 oxides are basic as the oxide ions accept H+ ions to become hydroxide ions in these reactions Calcium hydroxide is reasonably soluble in water. It is used in agriculture to neutralise acidic soils. If too much calcium hydroxide is added to the soil, excess will result in soils becoming too a ...
... Group 2 oxides are basic as the oxide ions accept H+ ions to become hydroxide ions in these reactions Calcium hydroxide is reasonably soluble in water. It is used in agriculture to neutralise acidic soils. If too much calcium hydroxide is added to the soil, excess will result in soils becoming too a ...
Bio-molecule
... Lipids are made mostly of carbon and hydrogen, and proteins contain nitrogen in addition to carbon, hydrogen and oxygen. ...
... Lipids are made mostly of carbon and hydrogen, and proteins contain nitrogen in addition to carbon, hydrogen and oxygen. ...
FINAL EXAM Review Sheet / Study Guide Honors Chemistry
... 2) Explain the difference between dissociation and dissolving. Use an example compound for each. ...
... 2) Explain the difference between dissociation and dissolving. Use an example compound for each. ...
Chap 24. Transition Metals and Coordination Compounds
... – The primary valence is the oxidation state on the central metal atom – the secondary valence is the number of molecules or ions directly bound to the metal atom, called the coordination number . • the primary valence is +3 • coordination number is 6 ...
... – The primary valence is the oxidation state on the central metal atom – the secondary valence is the number of molecules or ions directly bound to the metal atom, called the coordination number . • the primary valence is +3 • coordination number is 6 ...
BIOCHEMISTRY - Mexico Central School District
... Carbohydrates, Proteins, Lipids, Nucleic Acids ...
... Carbohydrates, Proteins, Lipids, Nucleic Acids ...
Preparation and transformation of competent bacteria: Calcium
... 12. Explain what refseq is. (You will have to search the NCBI Web Site to find this – go back to the home page and select NCBI Web Site from the drop down list). ...
... 12. Explain what refseq is. (You will have to search the NCBI Web Site to find this – go back to the home page and select NCBI Web Site from the drop down list). ...
Topic 2.4 Proteins Study Guide Amino acids are linked together by
... The amino acid sequence of polypeptides is coded for by genes. A protein may consist of a single ...
... The amino acid sequence of polypeptides is coded for by genes. A protein may consist of a single ...
Metalloprotein

Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large number of all proteins are part of this category.