Macromolecule Study Chart
... molecules to perform cellular functions. 2. Carbon skeletons of monosaccharides used as raw materials for making other organic molecules (i.e. amino acids, triglycerides, etc…). 3. linked to form polysaccharides 4. –ose suffix (glucose, fructose, etc…) ...
... molecules to perform cellular functions. 2. Carbon skeletons of monosaccharides used as raw materials for making other organic molecules (i.e. amino acids, triglycerides, etc…). 3. linked to form polysaccharides 4. –ose suffix (glucose, fructose, etc…) ...
Chapter 5 - Richsingiser.com
... 3. Treatment of 1 mol of FP with carboxypeptidase (cleaves at Cterminus of all residues) yields 2 mol of phenylalanine. ...
... 3. Treatment of 1 mol of FP with carboxypeptidase (cleaves at Cterminus of all residues) yields 2 mol of phenylalanine. ...
video slide
... backbone components Tertiary structure -- interactions between various side chains (R groups) Quaternary structure – proteins consisting of multiple polypeptide chains ...
... backbone components Tertiary structure -- interactions between various side chains (R groups) Quaternary structure – proteins consisting of multiple polypeptide chains ...
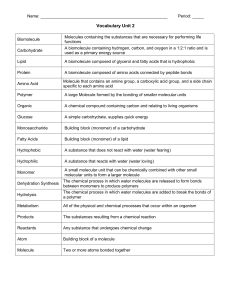
Name: Period: _____ Vocabulary Unit 2 Biomolecule Molecules
... A small molecular unit that can be chemically combined with other small molecular units to form a larger molecule ...
... A small molecular unit that can be chemically combined with other small molecular units to form a larger molecule ...
Honors BIOLOGY
... Consider the importance of the nitrogen bases especially adenine: DNA: the code for proteins RNA: carries the code to make proteins into the cytoplasm ATP: energy molecule of the cell All of these contain the double-ringed nitrogen base: adenine. Is it just a coincidence that caffeine and chocolate ...
... Consider the importance of the nitrogen bases especially adenine: DNA: the code for proteins RNA: carries the code to make proteins into the cytoplasm ATP: energy molecule of the cell All of these contain the double-ringed nitrogen base: adenine. Is it just a coincidence that caffeine and chocolate ...
Exam 2 Review - Iowa State University
... 4. The sum of oxidation numbers of all atoms in a compound equal the charge of that compound 1. Identify the oxidation number of each individual atom in the following equation. a. Which atom is being oxidized? Reduced? Zn (s) + H2SO4 (aq) ZnSO4 (aq) + H2 (g) ...
... 4. The sum of oxidation numbers of all atoms in a compound equal the charge of that compound 1. Identify the oxidation number of each individual atom in the following equation. a. Which atom is being oxidized? Reduced? Zn (s) + H2SO4 (aq) ZnSO4 (aq) + H2 (g) ...
Element or Compound?
... Copy down the following equations in your book. Underline the reactants and circle the products in each of the equations: Magnesium + Oxygen Iron + Oxygen ...
... Copy down the following equations in your book. Underline the reactants and circle the products in each of the equations: Magnesium + Oxygen Iron + Oxygen ...
Abstract
... Therefore, the aim of this study was to prepare a catalytically relevant bimetallic Nickel(II)complex with bridging NCP ligands and a chloro ligand at each metal center (Scheme 1). Reaction of a nickel(II) monomer with sodium azide did not yield the desired dimer as replacement of chloro ligands by ...
... Therefore, the aim of this study was to prepare a catalytically relevant bimetallic Nickel(II)complex with bridging NCP ligands and a chloro ligand at each metal center (Scheme 1). Reaction of a nickel(II) monomer with sodium azide did not yield the desired dimer as replacement of chloro ligands by ...
Amino acid sequence of phospholipase A from porcine pancreas
... been reported to be1,3: Alas, Arg 4, Asx23, Cysx4, Glx 7, Glye, His S, Ile 5, Leu 7, Lys,, Mete, Phe 5, Pro e, Serl0, Thr 7, Trp2, Tyr s, Val 2. However, subsequent determinations of tryptophan 4 and of half-cystine content by oxidation to cysteic acid 5 demonstrated the presence of only i tryptopha ...
... been reported to be1,3: Alas, Arg 4, Asx23, Cysx4, Glx 7, Glye, His S, Ile 5, Leu 7, Lys,, Mete, Phe 5, Pro e, Serl0, Thr 7, Trp2, Tyr s, Val 2. However, subsequent determinations of tryptophan 4 and of half-cystine content by oxidation to cysteic acid 5 demonstrated the presence of only i tryptopha ...
1. Overview
... • Coordinates can be extracted and viewed • Comparisons of structures allows identification of structural motifs • Proteins with similar functions and sequences = homologs ...
... • Coordinates can be extracted and viewed • Comparisons of structures allows identification of structural motifs • Proteins with similar functions and sequences = homologs ...
ANPS 019 Beneyto-Santonja 08-29
... Bonds tie one atom to another to create bigger chemical structure in the body Most types of bonds are made and broken by enzymes The role of enzymes Reactants (substrates) interact to yield a product by binding to the active site of the enzyme Enzymes serve as catalysts that promote chemical ...
... Bonds tie one atom to another to create bigger chemical structure in the body Most types of bonds are made and broken by enzymes The role of enzymes Reactants (substrates) interact to yield a product by binding to the active site of the enzyme Enzymes serve as catalysts that promote chemical ...
electron transport chain
... With the exception of coenzyme Q, all members of this chain are proteins. These may function as enzymes as is the case with the dehydrogenases, may contain iron as part of an iron–sulfur center, may be coordinated with a porphyrin ring as in the cytochromes, or may contain copper as does the cy ...
... With the exception of coenzyme Q, all members of this chain are proteins. These may function as enzymes as is the case with the dehydrogenases, may contain iron as part of an iron–sulfur center, may be coordinated with a porphyrin ring as in the cytochromes, or may contain copper as does the cy ...
Chapter 4
... Balance the non-H and non-O atoms Balance O by adding H2O where needed Balance H by adding H+ where needed Balance charge using eMultiply by coefficients until both e- are equal for each ½ reaction Add the ½ reactions together (cancel stuff) ...
... Balance the non-H and non-O atoms Balance O by adding H2O where needed Balance H by adding H+ where needed Balance charge using eMultiply by coefficients until both e- are equal for each ½ reaction Add the ½ reactions together (cancel stuff) ...
Flame Test Lab
... categorize substances, materials, and objects and how characteristics are used to categorize living things. GLE 1.1.1 Understand the atomic nature of matter, how it relates to physical and chemical properties and serves as the basis for the structure and use of the periodic table. Identify an unkn ...
... categorize substances, materials, and objects and how characteristics are used to categorize living things. GLE 1.1.1 Understand the atomic nature of matter, how it relates to physical and chemical properties and serves as the basis for the structure and use of the periodic table. Identify an unkn ...
Life Substances
... Define amino acids. How many amino acids are there? What makes one amino acid different from another? What do they look like? How are amino acids linked together? Define peptide bond What determines the kind of protein you have? Are hydrogen bonds part of the construction of proteins? Define enzyme. ...
... Define amino acids. How many amino acids are there? What makes one amino acid different from another? What do they look like? How are amino acids linked together? Define peptide bond What determines the kind of protein you have? Are hydrogen bonds part of the construction of proteins? Define enzyme. ...
A and P Practice Exam 01 (pdf 86.08kb)
... form water and in which the energy released along the way is used to generate ATP? a. glycolysis b. acetyl-CoA formation c. the Krebs cycle d. the electron transport chain 65. Pyruvic acid can be regarded as the end product of __________. a. glycolysis b. acetyl-CoA formation c. fermentation d. the ...
... form water and in which the energy released along the way is used to generate ATP? a. glycolysis b. acetyl-CoA formation c. the Krebs cycle d. the electron transport chain 65. Pyruvic acid can be regarded as the end product of __________. a. glycolysis b. acetyl-CoA formation c. fermentation d. the ...
Main types of Chemical Reactions
... 7. Carbonic acid decomposes into water and carbon dioxide. H2CO3 H2O + CO2 ...
... 7. Carbonic acid decomposes into water and carbon dioxide. H2CO3 H2O + CO2 ...
Available - Guru Ghasidas Vishwavidyalaya
... (c) High heat of vaporization (d) High dielectric constant (78.5 at 250 C) (Explain) 7. Write short note on denaturation of protein. All proteins begin their existence on a ribosome as a linear sequence of amino acid residues. This polypeptide must fold during and following synthesis to take up its ...
... (c) High heat of vaporization (d) High dielectric constant (78.5 at 250 C) (Explain) 7. Write short note on denaturation of protein. All proteins begin their existence on a ribosome as a linear sequence of amino acid residues. This polypeptide must fold during and following synthesis to take up its ...
Metalloprotein

Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large number of all proteins are part of this category.