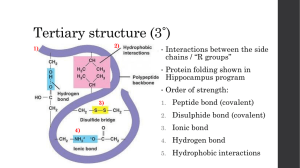
Proteins
... A few amino acids in a chain are called a polypeptide. A protein is usually composed of 50 to 400+ amino acids. Since part of the amino acid is lost during dehydration synthesis, we call the units of a protein amino acid residues. carbonyl carbon ...
... A few amino acids in a chain are called a polypeptide. A protein is usually composed of 50 to 400+ amino acids. Since part of the amino acid is lost during dehydration synthesis, we call the units of a protein amino acid residues. carbonyl carbon ...
Nucleic Acids - One Day Enrichment
... based on their chemical composition. • The four major groups of macromolecules found in living things are carbohydrates, lipids, nucleic acids, and proteins. ...
... based on their chemical composition. • The four major groups of macromolecules found in living things are carbohydrates, lipids, nucleic acids, and proteins. ...
introduction
... The hydrogen-bond networks created among water molecules change constantly on a subpicosecond time scale At any moment the H-bonds look like those in crystalline ice Solutes disrupt the H-bond networks ...
... The hydrogen-bond networks created among water molecules change constantly on a subpicosecond time scale At any moment the H-bonds look like those in crystalline ice Solutes disrupt the H-bond networks ...
Modeling with Toobers
... F. Compare your structure with structures made by others in the class. List 2 similarities and 2 differences you see? How do you explain the differences you observed? ...
... F. Compare your structure with structures made by others in the class. List 2 similarities and 2 differences you see? How do you explain the differences you observed? ...
No Slide Title
... Position 6 on the Beta Chain Hemoglobin C Glu to Lys at Position 6 on the Beta Chain ...
... Position 6 on the Beta Chain Hemoglobin C Glu to Lys at Position 6 on the Beta Chain ...
The structure of Matter
... O Compounds that contain only carbon and hydrogen are called hydrocarbons. O Two of the simplest hydrocarbons are methane and ethane. O Many hydrocarbons are used as fuels. ...
... O Compounds that contain only carbon and hydrogen are called hydrocarbons. O Two of the simplest hydrocarbons are methane and ethane. O Many hydrocarbons are used as fuels. ...
aptamers04
... New synthetic ribozymes, and DNAzymes Start with 1015 DNA molecules again Select for enzyme activity: E.g., cleaves itself off a solid support in the presence of Mg++ ...
... New synthetic ribozymes, and DNAzymes Start with 1015 DNA molecules again Select for enzyme activity: E.g., cleaves itself off a solid support in the presence of Mg++ ...
calculations-questions-part
... negative or neutral. The ligands may be neutral or negatively charged, but are never positively charged. The ligands must have a lone pair of electrons. In some complexes the central atom is neutral, as is the case with nickel carbonyl which is used in the Mond process for the purification of nickel ...
... negative or neutral. The ligands may be neutral or negatively charged, but are never positively charged. The ligands must have a lone pair of electrons. In some complexes the central atom is neutral, as is the case with nickel carbonyl which is used in the Mond process for the purification of nickel ...
Ionic compounds
... has the name of the metal ion written first. has the name of the nonmetal ion written second using the first syllable of its element name. followed by ide. has a space separating the name of the metal and nonmetal. ...
... has the name of the metal ion written first. has the name of the nonmetal ion written second using the first syllable of its element name. followed by ide. has a space separating the name of the metal and nonmetal. ...
Model Description Sheet
... develop breast cancer in their lifetime. Strikingly, many of these women share a significant genetic commonality. It has been shown that many breast cancer patients test positive for high levels of Estrogen Receptor (ERα), a protein that regulates the differentiation and maintenance of neural, skele ...
... develop breast cancer in their lifetime. Strikingly, many of these women share a significant genetic commonality. It has been shown that many breast cancer patients test positive for high levels of Estrogen Receptor (ERα), a protein that regulates the differentiation and maintenance of neural, skele ...
bio12_sm_07_3
... 9. Answers may vary. Presentation of findings should include: The human proteome project aims to generate a map of the protein molecular architecture of the human body, leading to the development of advanced diagnoses and treatments of diseases. The Human Proteome Organization uses molecular biologi ...
... 9. Answers may vary. Presentation of findings should include: The human proteome project aims to generate a map of the protein molecular architecture of the human body, leading to the development of advanced diagnoses and treatments of diseases. The Human Proteome Organization uses molecular biologi ...
Sample exam 1
... 6. Gastric juice has a pH of 1.5 and is produced by pumping HCl from blood plasma (pH 7.4) into the stomach. a. Calculate the free energy required to concentrate the H+ in 1 L of gastric juice at 37°C. For this problem, you can ignore the effects of the transmembrane electrical potential difference. ...
... 6. Gastric juice has a pH of 1.5 and is produced by pumping HCl from blood plasma (pH 7.4) into the stomach. a. Calculate the free energy required to concentrate the H+ in 1 L of gastric juice at 37°C. For this problem, you can ignore the effects of the transmembrane electrical potential difference. ...
cell respiration wilk hl ibdp
... • CoA comprises of [ adenine + ribose sugar + Pantothenic acid] • CoA is a carrier for Acetyl group into the Krebs cycle. NAD+ ...
... • CoA comprises of [ adenine + ribose sugar + Pantothenic acid] • CoA is a carrier for Acetyl group into the Krebs cycle. NAD+ ...
Chemistry of Life
... 2.4 Chemical Reactions Bonds break and form during chemical reactions. • Chemical reactions change substances into different ones by breaking and forming chemical bonds. – Reactants: changed during a chemical reaction. ...
... 2.4 Chemical Reactions Bonds break and form during chemical reactions. • Chemical reactions change substances into different ones by breaking and forming chemical bonds. – Reactants: changed during a chemical reaction. ...
Carbohydrates
... molecules that bond together to form long chains called polymers. The most common monosaccharides have the formula C6H12O6. The three most common monosaccharides are: Glucose: the only sugar living things can use for energy Fructose: the sugar found in fruit Galactose: a sugar found in milk. I ...
... molecules that bond together to form long chains called polymers. The most common monosaccharides have the formula C6H12O6. The three most common monosaccharides are: Glucose: the only sugar living things can use for energy Fructose: the sugar found in fruit Galactose: a sugar found in milk. I ...
Lecture 2.
... Interesting examples of carboranes are the extremely stable icosahedra closocarboranes. These boron-rich clusters exhibit unique organ mimetic properties with chemical reactivity matching classical organic molecules, yet structurally similar to metal-based inorganic and organ metallic species. A pro ...
... Interesting examples of carboranes are the extremely stable icosahedra closocarboranes. These boron-rich clusters exhibit unique organ mimetic properties with chemical reactivity matching classical organic molecules, yet structurally similar to metal-based inorganic and organ metallic species. A pro ...
Honors Chapter 11 Reactions
... numbers in front of formula distributes to numbers of atoms in formula specifies the relative number of moles and molecules involved in the reaction used to balance the equation ...
... numbers in front of formula distributes to numbers of atoms in formula specifies the relative number of moles and molecules involved in the reaction used to balance the equation ...
Updated Recovery Packet for Biochemistry.
... Ex. CO2 + H2OH2CO3 (allows blood to carry CO2) Energy in Reactions – may be released or absorbed 1. If release energy – usually spontaneous 2. If absorb energy – must use energy to start reaction e. Most organic reactions this type, so organisms need source of energy (sun, food) 3. Activation energ ...
... Ex. CO2 + H2OH2CO3 (allows blood to carry CO2) Energy in Reactions – may be released or absorbed 1. If release energy – usually spontaneous 2. If absorb energy – must use energy to start reaction e. Most organic reactions this type, so organisms need source of energy (sun, food) 3. Activation energ ...
FXM Rev 1 Key - Grande Cache Community High School
... the Planetary Atomic Model. hydrocarbons These are organic compounds that contain both carbon and hydrogen. Methane (CH4) is an example. Avogadro’s number This is a number that groups a very large amount of atoms or molecules to facilitate measurement and calculations in chemistry. It is the number ...
... the Planetary Atomic Model. hydrocarbons These are organic compounds that contain both carbon and hydrogen. Methane (CH4) is an example. Avogadro’s number This is a number that groups a very large amount of atoms or molecules to facilitate measurement and calculations in chemistry. It is the number ...
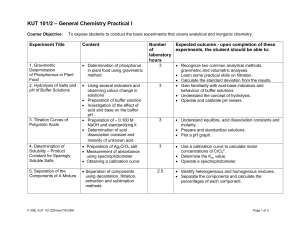
KUT 101/2 – General Chemistry Practical I
... • Preparation of Na2S2O3 solution and standardizing it • Determination of the oxidizing capacity of an unknown liquid bleach • Preparation of Cu(NO)3 and performing basic laboratory procedures • Reduction of copper with zinc • Preparation of ∼ 0.100 M NaOH and standardizing it. • Analysis of an unkn ...
... • Preparation of Na2S2O3 solution and standardizing it • Determination of the oxidizing capacity of an unknown liquid bleach • Preparation of Cu(NO)3 and performing basic laboratory procedures • Reduction of copper with zinc • Preparation of ∼ 0.100 M NaOH and standardizing it. • Analysis of an unkn ...
Metalloprotein

Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large number of all proteins are part of this category.