Chemistry to Remember
... of the element. The atom is made up of electrically charged particles: protons, neutrons, and electrons. Protons possess a positive charge and neutrons have no electrical charge. Protons and neutrons are contained within the nucleus of the atom and exert a positive charge. Negatively charged electro ...
... of the element. The atom is made up of electrically charged particles: protons, neutrons, and electrons. Protons possess a positive charge and neutrons have no electrical charge. Protons and neutrons are contained within the nucleus of the atom and exert a positive charge. Negatively charged electro ...
Chemistry-Biology Interface Symposium Frontiers at the
... APPLICATION OF TANDEM MASS SPECTROMETRY FOR QUANTIFYING LABELING DISTRIBUTIONS AND ESTIMATING METABOLIC FLUXES We have developed and introduced novel methodologies for MFA based on tandem MS and stable-isotope labeling experiments. We demonstrate that tandem MS provides more labeling information tha ...
... APPLICATION OF TANDEM MASS SPECTROMETRY FOR QUANTIFYING LABELING DISTRIBUTIONS AND ESTIMATING METABOLIC FLUXES We have developed and introduced novel methodologies for MFA based on tandem MS and stable-isotope labeling experiments. We demonstrate that tandem MS provides more labeling information tha ...
File - Ms. Daley Science
... 9) A young relative of yours has never had much energy. He goes to a doctor for help and is sent to the hospital for some tests. There they discover his mitochondria can use only fatty acids and amino acids for respiration, and his cells produce more lactic acid than normal. Of the following, which ...
... 9) A young relative of yours has never had much energy. He goes to a doctor for help and is sent to the hospital for some tests. There they discover his mitochondria can use only fatty acids and amino acids for respiration, and his cells produce more lactic acid than normal. Of the following, which ...
How to Measure the Similarity Between Protein
... residues are considered and described by a limited number of points, which are labeled according to pharmacophoric, geometric and/or chemical properties of their neighborhood. Second, the two resulting patterns are structurally aligned using the transformation that produces the maximum number of equ ...
... residues are considered and described by a limited number of points, which are labeled according to pharmacophoric, geometric and/or chemical properties of their neighborhood. Second, the two resulting patterns are structurally aligned using the transformation that produces the maximum number of equ ...
Answers to NHSCE 2002 Part A Page 1
... have the same molecular formulae but different structural formulae or connectivity. These isomers are called constitutional isomers (their former name was structural isomers). The molecular formula of (CH3)2O is C2H6O, and the alcohol with this molecular formula is C2H5OH. (The formula CH3-O-CH3 i ...
... have the same molecular formulae but different structural formulae or connectivity. These isomers are called constitutional isomers (their former name was structural isomers). The molecular formula of (CH3)2O is C2H6O, and the alcohol with this molecular formula is C2H5OH. (The formula CH3-O-CH3 i ...
The O 2
... the nature of the chemical bond and its application to the elucidation of the structure of complex ...
... the nature of the chemical bond and its application to the elucidation of the structure of complex ...
General Biology 115 Summer 2014
... In an attempt to treat this condition, several new drugs have been investigated. Drug A primarily blocks histamine receptors but also partially blocks acetylcholine receptors. Drug B blocks histamine receptors but has no effect on the acetylcholine receptors. Subjects with severe common colds, whose ...
... In an attempt to treat this condition, several new drugs have been investigated. Drug A primarily blocks histamine receptors but also partially blocks acetylcholine receptors. Drug B blocks histamine receptors but has no effect on the acetylcholine receptors. Subjects with severe common colds, whose ...
Jahn-Teller, Square Planar Complexes, Orbital
... the metal d orbitals are the frontier orbitals in most coordination complexes ...
... the metal d orbitals are the frontier orbitals in most coordination complexes ...
Balancing Chemical Equations
... Then all you do is list the atoms that are involved on each side of the arrow ...
... Then all you do is list the atoms that are involved on each side of the arrow ...
1 - Academics
... a) The complete transfer of a pair of e- between two atoms; b) The complete transfer of a single e- between two atoms; c) The sharing of a single e- between two atoms; d) When an electron falls into the nucleus of another atom. e) The sharing of a pair of e- between two atoms; 12. An ionic bond is b ...
... a) The complete transfer of a pair of e- between two atoms; b) The complete transfer of a single e- between two atoms; c) The sharing of a single e- between two atoms; d) When an electron falls into the nucleus of another atom. e) The sharing of a pair of e- between two atoms; 12. An ionic bond is b ...
Chemistry IGCSE
... enough so that the particles cannot move freely but can only vibrate. As a result, a solid has a stable, definite shape, and a definite volume. Solids can only change their shape by force, as when broken or cut. In crystalline solids, the particles (atoms, molecules, or ions) are packed in a regular ...
... enough so that the particles cannot move freely but can only vibrate. As a result, a solid has a stable, definite shape, and a definite volume. Solids can only change their shape by force, as when broken or cut. In crystalline solids, the particles (atoms, molecules, or ions) are packed in a regular ...
Recap: structure of ATP
... ATP is a source of energy Energy is released when ATP spits and forms ADP. The energy from this split is immediately available. A lot of the energy produced by cells ends up as heat (environment) therefore the body needs a continual source of energy. Cell respiration ...
... ATP is a source of energy Energy is released when ATP spits and forms ADP. The energy from this split is immediately available. A lot of the energy produced by cells ends up as heat (environment) therefore the body needs a continual source of energy. Cell respiration ...
Poster
... bacteria are needed. The Greenfield High School SMART (Students Modeling A Research Topic) Team is modeling the enzyme GatCAB using 3D printing technology. GatCAB is found in certain bacteria and archaea and could be a target for new antibiotics. During protein synthesis, ribosomes bring together am ...
... bacteria are needed. The Greenfield High School SMART (Students Modeling A Research Topic) Team is modeling the enzyme GatCAB using 3D printing technology. GatCAB is found in certain bacteria and archaea and could be a target for new antibiotics. During protein synthesis, ribosomes bring together am ...
Preferentially biotinylate N-terminal α
... Biotinylation reagents containing N-hydroxysuccinimide (NHS) esters are widely used to label proteins at primary amino groups (-NH2), which exist in the side chain of lysine residues and at the N-terminus of each polypeptide. With large proteins, labeling of several lysine residues and the N-terminu ...
... Biotinylation reagents containing N-hydroxysuccinimide (NHS) esters are widely used to label proteins at primary amino groups (-NH2), which exist in the side chain of lysine residues and at the N-terminus of each polypeptide. With large proteins, labeling of several lysine residues and the N-terminu ...
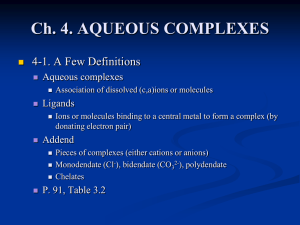
Ch. 3. KINETIC VS. EQUILIBRIUM MODELING
... Pieces of complexes (either cations or anions) Monodendate (Cl-), bidendate (CO32-), polydendate ...
... Pieces of complexes (either cations or anions) Monodendate (Cl-), bidendate (CO32-), polydendate ...
Word and Skeleton Equations Practice (ws Fall 2010)
... hydrogen, H2(g); nitrogen, N2(g); oxygen O2(g); fluorine, F2(g); chlorine Cl2(g); bromine, Br2(l); and iodine, I2(s). 1. Gaseous hydrogen and oxygen react to form gaseous water. 2. Solid sodium metal reacts with liquid water to form an aqueous solution of sodium hydroxide and hydrogen gas. 3. Solid ...
... hydrogen, H2(g); nitrogen, N2(g); oxygen O2(g); fluorine, F2(g); chlorine Cl2(g); bromine, Br2(l); and iodine, I2(s). 1. Gaseous hydrogen and oxygen react to form gaseous water. 2. Solid sodium metal reacts with liquid water to form an aqueous solution of sodium hydroxide and hydrogen gas. 3. Solid ...
Atomic Theory (2
... 2.) Of the diatomic substances, which has a triple bond? 3.) Describe the relationship between the number of bonds (single, double, or triple) and the strength of the bond. (In the first 2 questions, which has a stronger bond?) 4.) Draw the Lewis dot diagram for CH4. 5.) Draw the Lewis dot diagram f ...
... 2.) Of the diatomic substances, which has a triple bond? 3.) Describe the relationship between the number of bonds (single, double, or triple) and the strength of the bond. (In the first 2 questions, which has a stronger bond?) 4.) Draw the Lewis dot diagram for CH4. 5.) Draw the Lewis dot diagram f ...
carbohydrate metabolism
... dioxide involve many individual chemical reactions. These reactions occur in three different stages. These are: - initial break down of glucose to pyruvate in glycolysis, - further degradation of pyruvate to acetyl coenzyme A, and - finally complete oxidation of acetyl coenzyme A to water and carbon ...
... dioxide involve many individual chemical reactions. These reactions occur in three different stages. These are: - initial break down of glucose to pyruvate in glycolysis, - further degradation of pyruvate to acetyl coenzyme A, and - finally complete oxidation of acetyl coenzyme A to water and carbon ...
6.2 Assimilation of inorganic nitrogen
... 6.2.1.1 Biochemistry of N2 fixation Nitrogen reduction to ammonia : This is an exergonic reaction, but require a high activation energy due to the stable triple bond in N2. Nitrogen fixing microbes produce ‘nitrogenase’ reducing nitrogen under normal physiological conditions. 6.2.1.1.1 Nitroge ...
... 6.2.1.1 Biochemistry of N2 fixation Nitrogen reduction to ammonia : This is an exergonic reaction, but require a high activation energy due to the stable triple bond in N2. Nitrogen fixing microbes produce ‘nitrogenase’ reducing nitrogen under normal physiological conditions. 6.2.1.1.1 Nitroge ...
Chemical Calculations, Chemical Equations
... The most notable example of double replacement reactions is neutralization, a reaction between acids and bases (for our purposes, base is a compound containing a hydroxide ion). During neutralization, a salt and water are produced. ...
... The most notable example of double replacement reactions is neutralization, a reaction between acids and bases (for our purposes, base is a compound containing a hydroxide ion). During neutralization, a salt and water are produced. ...
Biochemistry Chp 3
... Lipids (CHO) 2x as many H as C, fewer O Proteins (CHONS) Nucleic Acids (CHONP) ...
... Lipids (CHO) 2x as many H as C, fewer O Proteins (CHONS) Nucleic Acids (CHONP) ...
Metalloprotein

Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large number of all proteins are part of this category.