AKUBOH OLIVIA 13/SCI03/001 BCH 413 METALLOENZYMES
... deprotonated in order for a donor atom (O, N, or S) to form a metal–ligand bond. Some metal ions coordinate to their binding sites in apo-metalloenzymes as simple aqua ions. For example, Zn2+ binds to Apo-carbonic anhydrase in a multi-dentate ligand reaction: the metal ion sheds coordinated water mo ...
... deprotonated in order for a donor atom (O, N, or S) to form a metal–ligand bond. Some metal ions coordinate to their binding sites in apo-metalloenzymes as simple aqua ions. For example, Zn2+ binds to Apo-carbonic anhydrase in a multi-dentate ligand reaction: the metal ion sheds coordinated water mo ...
Amino Acids Placemat
... together in a long linear polymer that folds into a compact shape following basic principles of chemistry and physics. ...
... together in a long linear polymer that folds into a compact shape following basic principles of chemistry and physics. ...
Predicting synthesis and decomposition reactions
... (MUCH more on this later!) • A reaction in which electrons are transferred from one atom to another is called an ____________________ reaction. ...
... (MUCH more on this later!) • A reaction in which electrons are transferred from one atom to another is called an ____________________ reaction. ...
Coordination compounds
... i. All Fe have 6, octahedral ii. cobalt which has 4 is tetrahedral 4. Ligands a. Can be neutral or ions, but must have lone pair of electrons to donate (lewis bases) i. Examples are: NH3, Cl-, H2O, OH-, CN-, NO2-1 b. Some ligands have more than one lone pair that they can donate. i. Bidentate: forms ...
... i. All Fe have 6, octahedral ii. cobalt which has 4 is tetrahedral 4. Ligands a. Can be neutral or ions, but must have lone pair of electrons to donate (lewis bases) i. Examples are: NH3, Cl-, H2O, OH-, CN-, NO2-1 b. Some ligands have more than one lone pair that they can donate. i. Bidentate: forms ...
Information regarding naming of Complex Ions Reference Sources: Naming Complex Ions
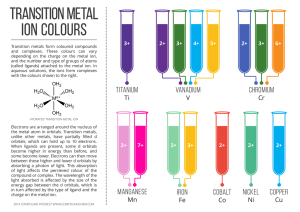
... For splitting of d orbitals due to ligands around central metal ion see: http://www.chemguide.co.uk/inorganic/complexions/colour2.html ...
... For splitting of d orbitals due to ligands around central metal ion see: http://www.chemguide.co.uk/inorganic/complexions/colour2.html ...
ap biology review guide big idea #2
... incorporate new nucleotides into the complementary strands. The cycle is then repeated over and over until there are millions of copies of the target DNA. ...
... incorporate new nucleotides into the complementary strands. The cycle is then repeated over and over until there are millions of copies of the target DNA. ...
Bioinorganic2
... directly to the iron in the heme as the sixth ligand(above the plane of the heme ring). Because hemoglobin has four heme units,each molecule can transport four oxygen molecules (and each blood cell hashundreds of thousands of hemoglobins in it). Once an oxygen molecule binds tothe first subunit's he ...
... directly to the iron in the heme as the sixth ligand(above the plane of the heme ring). Because hemoglobin has four heme units,each molecule can transport four oxygen molecules (and each blood cell hashundreds of thousands of hemoglobins in it). Once an oxygen molecule binds tothe first subunit's he ...
BIO 6.3 Carbon - Steinbach Science
... because amino acids can be put in various combinations The number of amino acids in protein chains determine the kind of protein Proteins are the building blocks of many structural components of orga ...
... because amino acids can be put in various combinations The number of amino acids in protein chains determine the kind of protein Proteins are the building blocks of many structural components of orga ...
The World of Chemistry
... 1. What are some of the ways mentioned that proteins are used in our bodies? ...
... 1. What are some of the ways mentioned that proteins are used in our bodies? ...
Unidentate, Bidentate and Multidentate Ligands
... In the examples we've already looked at, each ligand only forms one bond with the central metal ion to give the complex ion. Such a ligand is said to be unidentate. That means literally that it only has one tooth! It only has one pair of electrons that it can use to bond to the metal - any other lon ...
... In the examples we've already looked at, each ligand only forms one bond with the central metal ion to give the complex ion. Such a ligand is said to be unidentate. That means literally that it only has one tooth! It only has one pair of electrons that it can use to bond to the metal - any other lon ...
Protein Engineering
... •Increase the efficiency of enzyme-catalyzed reactions • Eliminate the need for cofactor in enzymatic reaction •Change substrate binding site to increase specificity •Change the thermal tolerance •Change the pH stability •Increase proteins resistance to proteases (purification) •Signal sequences - s ...
... •Increase the efficiency of enzyme-catalyzed reactions • Eliminate the need for cofactor in enzymatic reaction •Change substrate binding site to increase specificity •Change the thermal tolerance •Change the pH stability •Increase proteins resistance to proteases (purification) •Signal sequences - s ...
this lecture as PDF here
... Enzymes require an additional non-protein component to carry out its catalytic functions. ...
... Enzymes require an additional non-protein component to carry out its catalytic functions. ...
Lesson
... * Amino acid structure & properties * Stages involved with formation of proteins * Primary, secondary, tertiary & quaternary structures ...
... * Amino acid structure & properties * Stages involved with formation of proteins * Primary, secondary, tertiary & quaternary structures ...
Coordination Compounds
... • Complex ion: can be cation or anion; contains the transition metal and usually is in brackets. • Ligands: neutral molecule or ion having a lone pair of electrons that forms a coordinate covalent bond to a metal ion; usually dissociate when aqueous. Ligands in the spectrochemical series show which ...
... • Complex ion: can be cation or anion; contains the transition metal and usually is in brackets. • Ligands: neutral molecule or ion having a lone pair of electrons that forms a coordinate covalent bond to a metal ion; usually dissociate when aqueous. Ligands in the spectrochemical series show which ...
Measurement of Protein Molecular Weight using MALDI MS
... To calculate the molecular weight of the protein, the measured m/z value of charge state, n, is multiplied by n and then n protons (n * 1.0079) are subtracted to give the measured molecular weight. ...
... To calculate the molecular weight of the protein, the measured m/z value of charge state, n, is multiplied by n and then n protons (n * 1.0079) are subtracted to give the measured molecular weight. ...
Serine Proteases Teaching Exercises
... c. Find phenylalanine, tyrosine, tryptophan and methionine. 4. When these enzymes are made, they are initially in an inactive precursor state, called zymogens. In order for the enzymes to become active, they will be cleaved to arrange the linear amino acid sequence in such a fashion as to orient thr ...
... c. Find phenylalanine, tyrosine, tryptophan and methionine. 4. When these enzymes are made, they are initially in an inactive precursor state, called zymogens. In order for the enzymes to become active, they will be cleaved to arrange the linear amino acid sequence in such a fashion as to orient thr ...
Polyatomic Ions (Memorize for Wednesday, January 31
... EX – H2SO4: sulfate ion changes to sulfuric acid Polyatomic ions ending in –ite form acids with an –ous ending EX – HClO2: chlorite ion changes to chlorous acid The number of hydrogen atoms equals the charge on the polyatomic ion EX – H3PO4: H+1 x 3 = PO4-3 ...
... EX – H2SO4: sulfate ion changes to sulfuric acid Polyatomic ions ending in –ite form acids with an –ous ending EX – HClO2: chlorite ion changes to chlorous acid The number of hydrogen atoms equals the charge on the polyatomic ion EX – H3PO4: H+1 x 3 = PO4-3 ...
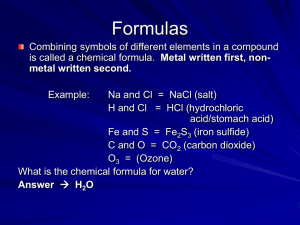
Formula Notes and Criss Cross Method
... Written below and after the symbol of the atom to indicate the number of atoms of that element in the compound. if no subscript, then = 1 atom Example: H2O ...
... Written below and after the symbol of the atom to indicate the number of atoms of that element in the compound. if no subscript, then = 1 atom Example: H2O ...
Metalloprotein

Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large number of all proteins are part of this category.