Graphene-Catalyzed Direct Friedel–Crafts Alkylation Reactions
... toxic waste, and suffers from limited natural abundance of transition metals.18 Transition-metal-free procedures have also been reported;19,20 however, these methods are not general and suffer from harsh reaction conditions, further limiting their substrate scope. Given the importance of alkylarenes i ...
... toxic waste, and suffers from limited natural abundance of transition metals.18 Transition-metal-free procedures have also been reported;19,20 however, these methods are not general and suffer from harsh reaction conditions, further limiting their substrate scope. Given the importance of alkylarenes i ...
Catalytic decomposition of N2O over Rh/Zn–Al2O3 catalysts
... lead to more active catalysts due to the improved dispersion of Rh species.28 Parres-Esclapez et al. found that Sr can promote the activity of Rh/Al2O3 due to the improved dispersion and reducibility of Rh species.29 Zhao et al. reported that Rh/SiO2–Al2O3 shows high activity, because oxygen desorpt ...
... lead to more active catalysts due to the improved dispersion of Rh species.28 Parres-Esclapez et al. found that Sr can promote the activity of Rh/Al2O3 due to the improved dispersion and reducibility of Rh species.29 Zhao et al. reported that Rh/SiO2–Al2O3 shows high activity, because oxygen desorpt ...
Catalysts Containing Depleted Uranium Compounds
... The prevailing consumption of uranium in the form of uranium dioxide is made by the enterprises of atomic power engineering, thus oxygen-containing compounds of uranium were investigated most completely with the purpose of manufacturing nuclear fuel. At the same time, due to the aforementioned varie ...
... The prevailing consumption of uranium in the form of uranium dioxide is made by the enterprises of atomic power engineering, thus oxygen-containing compounds of uranium were investigated most completely with the purpose of manufacturing nuclear fuel. At the same time, due to the aforementioned varie ...
Chemistry
... advanced computerised equipment available in many analytical laboratories. The editors have built further on the work of Dr Vogel, modernising the approach while retaining the analytical concepts and ideas which were built into the original work. This new edition has been extensively revised to take ...
... advanced computerised equipment available in many analytical laboratories. The editors have built further on the work of Dr Vogel, modernising the approach while retaining the analytical concepts and ideas which were built into the original work. This new edition has been extensively revised to take ...
Proposed syllabus and Scheme of Examination B.Sc. (Program) with
... 3. Estimation of water of crystallization in Mohr’s salt by titrating with KMnO4. 4. Estimation of Fe (II) ions by titrating it with K2Cr2O7 using internal indicator. 5. Estimation of Cu (II) ions iodometrically using Na2S2O3. Section B: Organic Chemistry 1. Detection of extra elements (N, S, Cl, Br ...
... 3. Estimation of water of crystallization in Mohr’s salt by titrating with KMnO4. 4. Estimation of Fe (II) ions by titrating it with K2Cr2O7 using internal indicator. 5. Estimation of Cu (II) ions iodometrically using Na2S2O3. Section B: Organic Chemistry 1. Detection of extra elements (N, S, Cl, Br ...
Document
... Amines as Resolving Agents A type of amine called alkaloids is available from plant sources. Many are chiral and occur in single enantiomer form. Some of these compounds are pharmacologically important, e. g., quinine and atropine. Others have dangerous natures, e. g., morphine and strychnine. Some ...
... Amines as Resolving Agents A type of amine called alkaloids is available from plant sources. Many are chiral and occur in single enantiomer form. Some of these compounds are pharmacologically important, e. g., quinine and atropine. Others have dangerous natures, e. g., morphine and strychnine. Some ...
Chapter Seven - U of L Class Index
... The Sn 1 mechanism involves the formation of a carbocation intermediate in the ratedetermining step. 3°, benzylic and allylic substrates undergo Sn 1 reaction because they form relatively stable carbocations. ...
... The Sn 1 mechanism involves the formation of a carbocation intermediate in the ratedetermining step. 3°, benzylic and allylic substrates undergo Sn 1 reaction because they form relatively stable carbocations. ...
幻灯片 1
... would be given the formula RO. But Williamson, by his ether synthesis, showed that mixed ethers, with two different alkyl groups, could be prepared. Ethers thus has to have the water-type formula ROR', and oxygen had the equivalent weight of 8 but the atomic weight of 16. By this type of argument he ...
... would be given the formula RO. But Williamson, by his ether synthesis, showed that mixed ethers, with two different alkyl groups, could be prepared. Ethers thus has to have the water-type formula ROR', and oxygen had the equivalent weight of 8 but the atomic weight of 16. By this type of argument he ...
M.Sc. Chemistry : Syllabus (CBCS)
... determination of reaction mechanism. To understand the mechanism of nucleophilic and electrophilic substitution reactions. UNIT-I: STEREOCHEMISTRY Optical activity and chirality, Classification ofchiral moleculesas asymmetricand dissymmetric. A brief Study of dissymmetry of allenes, biphenyls, spiro ...
... determination of reaction mechanism. To understand the mechanism of nucleophilic and electrophilic substitution reactions. UNIT-I: STEREOCHEMISTRY Optical activity and chirality, Classification ofchiral moleculesas asymmetricand dissymmetric. A brief Study of dissymmetry of allenes, biphenyls, spiro ...
13-Elimination Reactions
... reaction mechanisms and compares them with the stereochemistry of the SN1 and SN2 reaction mechanisms, which are covered in Chapter 12. The E1 reaction mechanism is a two-step process that, as with the SN1 mechanism, usually loses all the stereochemical information of the substrate as the reaction p ...
... reaction mechanisms and compares them with the stereochemistry of the SN1 and SN2 reaction mechanisms, which are covered in Chapter 12. The E1 reaction mechanism is a two-step process that, as with the SN1 mechanism, usually loses all the stereochemical information of the substrate as the reaction p ...
Carbonyl Condensation Reactions
... carbonyl compound becomes the nucleophilic enolate and which reacts at the electrophilic carbonyl carbon. The strategy of a directed aldol reaction is as follows: [1] Prepare the enolate of one carbonyl component with LDA. [2] Add the second carbonyl compound (the electrophile) to this enolate. Beca ...
... carbonyl compound becomes the nucleophilic enolate and which reacts at the electrophilic carbonyl carbon. The strategy of a directed aldol reaction is as follows: [1] Prepare the enolate of one carbonyl component with LDA. [2] Add the second carbonyl compound (the electrophile) to this enolate. Beca ...
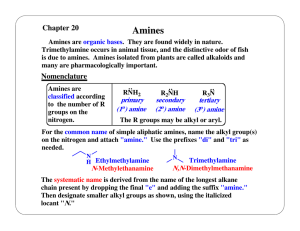
Amines
... This is because -NH2, -NHR2 and -NR2 are very strong activators and are ortho, paradirecting. However the basicity of the amino group means it is unsuitable for reactions with acids (e.g. H2SO4 or AlCl3) such as nitration, sulfonation and Friedel-Crafts alkylation or acylation. Polysubstitution can ...
... This is because -NH2, -NHR2 and -NR2 are very strong activators and are ortho, paradirecting. However the basicity of the amino group means it is unsuitable for reactions with acids (e.g. H2SO4 or AlCl3) such as nitration, sulfonation and Friedel-Crafts alkylation or acylation. Polysubstitution can ...
Orbitals
... The Wittig Reaction Wittig reaction yields a single, pure alkene of defined structure • Grignard reaction yields two products • Most-substituted double bond is the major product • Wittig reaction yields only one product • Less-substituted double bond is the sole product ...
... The Wittig Reaction Wittig reaction yields a single, pure alkene of defined structure • Grignard reaction yields two products • Most-substituted double bond is the major product • Wittig reaction yields only one product • Less-substituted double bond is the sole product ...
Under Choice Based Credit System Proposed syllabus and Scheme of Examination
... carbon atoms). Configuration: Geometrical and Optical isomerism; Enantiomerism, Diastereomerism and Meso compounds). Threo and erythro; D and L; cis - trans nomenclature; CIP Rules: R/ S (for upto 2 chiral carbon atoms) and E / Z Nomenclature (for upto two C=C systems). (10 Lectures) Aliphatic Hydro ...
... carbon atoms). Configuration: Geometrical and Optical isomerism; Enantiomerism, Diastereomerism and Meso compounds). Threo and erythro; D and L; cis - trans nomenclature; CIP Rules: R/ S (for upto 2 chiral carbon atoms) and E / Z Nomenclature (for upto two C=C systems). (10 Lectures) Aliphatic Hydro ...
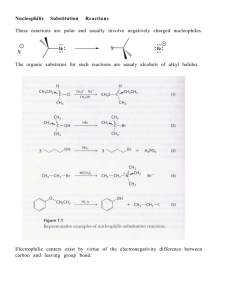
Chapter 8 I. Nucleophilic Substitution
... True (A) / False (B) A racemic mixture of (R- ) and (S- )-2bromobutane produces an optically active product. ...
... True (A) / False (B) A racemic mixture of (R- ) and (S- )-2bromobutane produces an optically active product. ...
Aldehydes, Ketones, & Chiral Molecules
... Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings ...
... Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings ...
The Grob Fragmentation
... -Grob fragmentation: Fragmentation substrates are typically 1,3diheterofunctionalized compounds featuring a nucelophilic atom with a negative ...
... -Grob fragmentation: Fragmentation substrates are typically 1,3diheterofunctionalized compounds featuring a nucelophilic atom with a negative ...
Scope and Limitations - Organic Reactions Wiki
... groups and lack of over-oxidation products.4 Researchers from the UpJohn company reported a convenient and reliable procedure for dihydroxylation that involved substoichiometric amounts of OsO4 (typically 5 mol %) and N-methymorpholine-N-oxide (NMO) as a stoichiometric co-oxidant. This landmark pape ...
... groups and lack of over-oxidation products.4 Researchers from the UpJohn company reported a convenient and reliable procedure for dihydroxylation that involved substoichiometric amounts of OsO4 (typically 5 mol %) and N-methymorpholine-N-oxide (NMO) as a stoichiometric co-oxidant. This landmark pape ...
Chemical Properties of Monocyclic Aromatic Hydrocarbons(5)
... Further electrophilic substitution of a disubstituted benzene is governed by the same resonance and inductive effects just discussed. The only difference is that it’s necessary to consider the additive effects of two different groups. In practice, three rules are usually sufficient: Rule 1. If the d ...
... Further electrophilic substitution of a disubstituted benzene is governed by the same resonance and inductive effects just discussed. The only difference is that it’s necessary to consider the additive effects of two different groups. In practice, three rules are usually sufficient: Rule 1. If the d ...
Factors influencing ring closure through olefin metathesis – A
... or radical species. Common rings such as 5–7 membered ones are easily available by these methods. However, formation of medium or large rings by these methods either proceeds with low yields or does not proceed at all due to unfavourable enthalpic and entropic factors. In recent years, olefin metath ...
... or radical species. Common rings such as 5–7 membered ones are easily available by these methods. However, formation of medium or large rings by these methods either proceeds with low yields or does not proceed at all due to unfavourable enthalpic and entropic factors. In recent years, olefin metath ...
Organic Synthesis II
... Mechanisms for many oxidation reactions (even well-known ones) are significantly more complex than drawn throughout this course (and in many cases are not known or understood). Some are based on factual mechanistic data; some should be treated more as a mnemonic than explanation. ...
... Mechanisms for many oxidation reactions (even well-known ones) are significantly more complex than drawn throughout this course (and in many cases are not known or understood). Some are based on factual mechanistic data; some should be treated more as a mnemonic than explanation. ...
The catalytic function of a silica gel-immobilized Mn(II)
... range of alkenes [6]. Manganese porphyrin complexes have been extensively investigated as models for enzymes and used in catalysis with various single oxygen atom donors including H2 O2 [7]. Alkene epoxidation reactions catalyzed by manganese complexes in homogeneous and heterogeneous media and the ...
... range of alkenes [6]. Manganese porphyrin complexes have been extensively investigated as models for enzymes and used in catalysis with various single oxygen atom donors including H2 O2 [7]. Alkene epoxidation reactions catalyzed by manganese complexes in homogeneous and heterogeneous media and the ...
Enantioselective synthesis

Enantioselective synthesis, also called chiral synthesis or asymmetric synthesis, is defined by IUPAC as: a chemical reaction (or reaction sequence) in which one or more new elements of chirality are formed in a substrate molecule and which produces the stereoisomeric (enantiomeric or diastereoisomeric) products in unequal amounts.Put more simply: it is the synthesis of a compound by a method that favors the formation of a specific enantiomer or diastereomer.Enantioselective synthesis is a key process in modern chemistry and is particularly important in the field of pharmaceuticals, as the different enantiomers or diastereomers of a molecule often have different biological activity.