The first practical method for asymmetric epoxidation
... ’ ~ ~ (c 1.5, CHC13). of 2(S),3(S)-epoxygeraniol, [ c u ]-6.36’ Analysis of this material as the MTPA ester” gave an enantiomeric excess (ee) of >95% whereas analysis of the derived epoxy acetate by using Eu(hfbc)3 chiral shift reagent gave 94% ee. The “typical procedure” given for geraniol has a li ...
... ’ ~ ~ (c 1.5, CHC13). of 2(S),3(S)-epoxygeraniol, [ c u ]-6.36’ Analysis of this material as the MTPA ester” gave an enantiomeric excess (ee) of >95% whereas analysis of the derived epoxy acetate by using Eu(hfbc)3 chiral shift reagent gave 94% ee. The “typical procedure” given for geraniol has a li ...
alcohols - GC12chem
... Secondary alcohol: the C atom with the OH group attached is attached to two other C atoms CH3CH2CHOHCH3 Tertiary alcohol: the C atom with the OH group attached is attached to three other C atoms CH3CH2C(CH3)OHCH3 12 Chemistry 2.5 organic chemistry CR 07 ...
... Secondary alcohol: the C atom with the OH group attached is attached to two other C atoms CH3CH2CHOHCH3 Tertiary alcohol: the C atom with the OH group attached is attached to three other C atoms CH3CH2C(CH3)OHCH3 12 Chemistry 2.5 organic chemistry CR 07 ...
• Pergamon
... 2-methyl group occurred within 5 min. Then, addition of the a-free pyrrole, 2-benzyloxycarbonyl-3,4-dimethylpyrrole 9, into the same reaction mixture resulted in in situ coupling to provide (after ...
... 2-methyl group occurred within 5 min. Then, addition of the a-free pyrrole, 2-benzyloxycarbonyl-3,4-dimethylpyrrole 9, into the same reaction mixture resulted in in situ coupling to provide (after ...
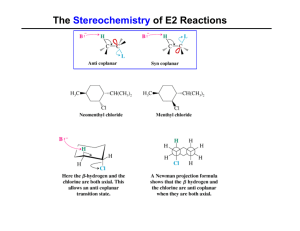
The Stereochemistry of E2 Reactions
... Hammond-Leffler Postulate: the transition state for a step that is downhill in energy should show a strong resemblance to the reactant of that step. ...
... Hammond-Leffler Postulate: the transition state for a step that is downhill in energy should show a strong resemblance to the reactant of that step. ...
Alcohols, Diols And Triols
... The tertiary alcohol tert-butyl alcohol is not oxidized by acidic sodium dichromate solution. The carbon atom bearing the hydroxyl group in this alcohol does not have a hydrogen atom on it. Here, too, carbon-carbon bonds would have to be broken to allow oxidation to take place. ...
... The tertiary alcohol tert-butyl alcohol is not oxidized by acidic sodium dichromate solution. The carbon atom bearing the hydroxyl group in this alcohol does not have a hydrogen atom on it. Here, too, carbon-carbon bonds would have to be broken to allow oxidation to take place. ...
Discussion Worksheet #10 Formation of Alcohols Skill 1: Functional
... Problem 5. Plan two alternative syntheses involving Grignard reagents for each of these compounds. If two syntheses are not possible, explain. In which cases could an ester be used as the starting material? 1. xs CH3Ch2MgI 2. H3O+ ...
... Problem 5. Plan two alternative syntheses involving Grignard reagents for each of these compounds. If two syntheses are not possible, explain. In which cases could an ester be used as the starting material? 1. xs CH3Ch2MgI 2. H3O+ ...
Chapter 11 - Alcohols and Ethers1
... - Ethers have boiling points that are roughly comparable with those of hydrocarbons of the same molecular weight (unlike alcohols, which have higher) - Ethers can form hydrogen bonds with other molecules (eg: ...
... - Ethers have boiling points that are roughly comparable with those of hydrocarbons of the same molecular weight (unlike alcohols, which have higher) - Ethers can form hydrogen bonds with other molecules (eg: ...
IOSR Journal of Applied Chemistry (IOSR-JAC) ISSN: 2278-5736.
... In this letter, we wish to report a mild efficient and ecofriendly method to protect 1,2 or 1,3 diols as their acetonides with acetone and a cation exchange resin. The reaction can be carried out in toluene or without toluene as a solvent. The selectivity and versatility of the of the thermal solven ...
... In this letter, we wish to report a mild efficient and ecofriendly method to protect 1,2 or 1,3 diols as their acetonides with acetone and a cation exchange resin. The reaction can be carried out in toluene or without toluene as a solvent. The selectivity and versatility of the of the thermal solven ...
Chapter 1 Structure and Bonding
... 1) Reduction reactions can be reversed to give the aldehydes or ketones 2) Oxidizing Reagent is Cr(VI) a) (Na2Cr2O7 or K2Cr2O7 or CrO3) and H2SO4 and H2O R' ...
... 1) Reduction reactions can be reversed to give the aldehydes or ketones 2) Oxidizing Reagent is Cr(VI) a) (Na2Cr2O7 or K2Cr2O7 or CrO3) and H2SO4 and H2O R' ...
CHAPTER-6 DEHYDROHALOGENATION OF ALKYL HALIDES
... – Approach to carbon is extremely hindered and elimination predominates especially at high temperatures ...
... – Approach to carbon is extremely hindered and elimination predominates especially at high temperatures ...
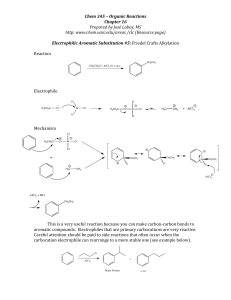
EAS Friedel-Crafts Alkylation
... The Friedel-‐Crafts reaction only requires catalytic amounts of the Lewis acid because it is recycled through the reaction. There is a major disadvantage of the alkylation reaction and that is over-‐ alkyla ...
... The Friedel-‐Crafts reaction only requires catalytic amounts of the Lewis acid because it is recycled through the reaction. There is a major disadvantage of the alkylation reaction and that is over-‐ alkyla ...
2d Oxidation of Food Homework
... 15. The quantity of alcohol present after a fermentation reaction is called the % alcohol by volume. This can be calculated from measurements taken using an instrument called a hydrometer. The hydrometer is floated in the liquid sample, before and after fermentation, to measure its specific gravity. ...
... 15. The quantity of alcohol present after a fermentation reaction is called the % alcohol by volume. This can be calculated from measurements taken using an instrument called a hydrometer. The hydrometer is floated in the liquid sample, before and after fermentation, to measure its specific gravity. ...
Oxidising Alcohols
... The pH paper only changed to red in the tube containing the primary alcohol. Only the primary alcohol forms an acid. ...
... The pH paper only changed to red in the tube containing the primary alcohol. Only the primary alcohol forms an acid. ...
Table
... Condensation Reaction Pathway to other compounds Ester+NaOH sodium salt of acid+ alcohol Hydrolysis; saponification Preparation Amines: RX+NH3 amine + HX RX+R2NH amine +HX Amide +H2Ocarboxyic acid + amine (hydrolysis reaction) Amides Carboxylic acid + amine amide + H2O (condensation reaction) ...
... Condensation Reaction Pathway to other compounds Ester+NaOH sodium salt of acid+ alcohol Hydrolysis; saponification Preparation Amines: RX+NH3 amine + HX RX+R2NH amine +HX Amide +H2Ocarboxyic acid + amine (hydrolysis reaction) Amides Carboxylic acid + amine amide + H2O (condensation reaction) ...
ALCOHOLS
... The oxidising agent changes colour from orange to green. 3. Tertiary alcohols are not oxidised. The oxidising agent stays orange. The reaction with acidified potassium dichromate(VI) distinguishes tertiary alcohols from primary and secondary alcohols. ...
... The oxidising agent changes colour from orange to green. 3. Tertiary alcohols are not oxidised. The oxidising agent stays orange. The reaction with acidified potassium dichromate(VI) distinguishes tertiary alcohols from primary and secondary alcohols. ...
Synthesis of a Family of Chiral Asymmetric Schiff - Blogs at H-SC
... Condensation reactions of carbonyl compounds are an important class of such reactions. Chiral organometallic compounds have been shown in some cases to act as catalysts to give condensation products in high yields and with high ...
... Condensation reactions of carbonyl compounds are an important class of such reactions. Chiral organometallic compounds have been shown in some cases to act as catalysts to give condensation products in high yields and with high ...
Document
... 15.5: Preparation of Diols - Vicinal diols have hydroxyl groups on adjacent carbons (1,2-diols, vic-diols, glycols) Dihydroxylation: formal addition of HO-OH across the -bond of an alkene to give a 1,2-diol. This is an overall oxidation. ...
... 15.5: Preparation of Diols - Vicinal diols have hydroxyl groups on adjacent carbons (1,2-diols, vic-diols, glycols) Dihydroxylation: formal addition of HO-OH across the -bond of an alkene to give a 1,2-diol. This is an overall oxidation. ...
alcohols - A-Level Chemistry
... Suggest how methanol could be made in two steps from methane. i) ...
... Suggest how methanol could be made in two steps from methane. i) ...
Ch 8 Lecture 1
... e) Organic molecules with polar functional groups (-OH, -NH2, -CO2H) have much higher water solubilities 2) The longer the alkyl group, the less water soluble the alcohol (more soluble in hydrocarbons—like dissolves like) 3) MeOH and EtOH are very similar to water as solvents: many salts will dissol ...
... e) Organic molecules with polar functional groups (-OH, -NH2, -CO2H) have much higher water solubilities 2) The longer the alkyl group, the less water soluble the alcohol (more soluble in hydrocarbons—like dissolves like) 3) MeOH and EtOH are very similar to water as solvents: many salts will dissol ...
File
... Zealand police used ‘breathalysers’ to measure breath alcohol. The subject blew through this tube to fill a bag. The crystals of potassium dichromate in the tube turned green if ethanol was present. If there was sufficient ethanol in the breath to turn crystals beyond the line green, then the person ...
... Zealand police used ‘breathalysers’ to measure breath alcohol. The subject blew through this tube to fill a bag. The crystals of potassium dichromate in the tube turned green if ethanol was present. If there was sufficient ethanol in the breath to turn crystals beyond the line green, then the person ...
Slide 1
... A crown ether specifically binds certain metal ions or organic molecules to form a host–guest complex, an example of molecular recognition ...
... A crown ether specifically binds certain metal ions or organic molecules to form a host–guest complex, an example of molecular recognition ...
Alcohols Oxidation by oxygen O2 in presence of
... activities. Also the benzylic alcohols type 2 such as1phenyl alcohols and benzhydrol has been oxidized slower than the benzyl alcohols type i.. In addition allylic alcohol such as cinnamyl alcohol are selectively changed in to the relevant aldehyde without the inter mention of carbon- carbon double ...
... activities. Also the benzylic alcohols type 2 such as1phenyl alcohols and benzhydrol has been oxidized slower than the benzyl alcohols type i.. In addition allylic alcohol such as cinnamyl alcohol are selectively changed in to the relevant aldehyde without the inter mention of carbon- carbon double ...
Kinetic resolution

In organic chemistry, kinetic resolution is a means of differentiating two enantiomers in a racemic mixture. In kinetic resolution, two enantiomers react with different reaction rates in a chemical reaction with a chiral catalyst or reagent, resulting in an enantioenriched sample of the less reactive enantiomer. As opposed to chiral resolution, kinetic resolution does not rely on different physical properties of diastereomeric products, but rather on the different chemical properties of the racemic starting materials. This enantiomeric excess (ee) of the unreacted starting material continually rises as more product is formed, reaching 100% just before full completion of the reaction. Kinetic resolution relies upon differences in reactivity between enantiomers or enantiomeric complexes. Kinetic resolution is a concept in organic chemistry and can be used for the preparation of chiral molecules in organic synthesis. Kinetic resolution reactions utilizing purely synthetic reagents and catalysts are much less common than the use of enzymatic kinetic resolution in application towards organic synthesis, although a number of useful synthetic techniques have been developed in the past 30 years.