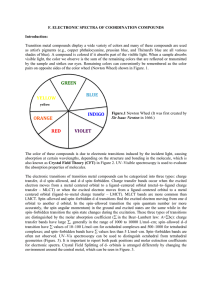
F. ELECTRONIC SPECTRA OF COORDINATION COMPOUNDS
... transfer, d–d spin–allowed, and d–d spin–forbidden. Charge transfer bands occur when the excited electron moves from a metal centered orbital to a ligand–centered orbital (metal–to–ligand charge transfer – MLCT) or when the excited electron moves from a ligand–centered orbital to a metal centered or ...
... transfer, d–d spin–allowed, and d–d spin–forbidden. Charge transfer bands occur when the excited electron moves from a metal centered orbital to a ligand–centered orbital (metal–to–ligand charge transfer – MLCT) or when the excited electron moves from a ligand–centered orbital to a metal centered or ...
SYNTHESIS, PHYSICO-CHEMICAL STUDIES AND ANTIMICROBIAL EVALUATION OF NOVEL 2- H COMPLEXES Research Article
... The synthesis and characterization of some new 2-(substituted aryl)-1H-benzo[d]thiazole ligands and their transition metal complexes are reported. All metal complexes were synthesized from the transition metal halide salts and 2-(substituted aryl)-1H-benzo[d]thiazoles in alcohol as a solvent at room ...
... The synthesis and characterization of some new 2-(substituted aryl)-1H-benzo[d]thiazole ligands and their transition metal complexes are reported. All metal complexes were synthesized from the transition metal halide salts and 2-(substituted aryl)-1H-benzo[d]thiazoles in alcohol as a solvent at room ...
Worked_Examples
... a. The only attractions between molecules of alkanes such as pentane are dispersion forces. With no dipole–dipole attractions or hydrogen bonds, pentane has the lowest boiling point of the three compounds. With a polar carbonyl group, butanone molecules form dipole–dipole attractions, but no hydroge ...
... a. The only attractions between molecules of alkanes such as pentane are dispersion forces. With no dipole–dipole attractions or hydrogen bonds, pentane has the lowest boiling point of the three compounds. With a polar carbonyl group, butanone molecules form dipole–dipole attractions, but no hydroge ...
a contribution to weaken health problems in bolivia: bioactive
... physiochemical processes. Iron and zinc are classified as essential metals without which life cannot sustain. Cadmium is a toxic metal which is harmful even at very low concentration. The normal and toxic level in human diet (mg/day) are (0.007 – 0.3; 3.0 – 330.0), (6.0 – 10.0; 200.0) (5.0 – 40.0; 1 ...
... physiochemical processes. Iron and zinc are classified as essential metals without which life cannot sustain. Cadmium is a toxic metal which is harmful even at very low concentration. The normal and toxic level in human diet (mg/day) are (0.007 – 0.3; 3.0 – 330.0), (6.0 – 10.0; 200.0) (5.0 – 40.0; 1 ...
Losing and Gaining Electrons
... (i.e., the solid angle) that the ligand occupies around the imaginary first coordination sphere. Both of these factors can be incorporated into the concept of the “% buried volume”, the volume of the ligand inside the coordination sphere. ...
... (i.e., the solid angle) that the ligand occupies around the imaginary first coordination sphere. Both of these factors can be incorporated into the concept of the “% buried volume”, the volume of the ligand inside the coordination sphere. ...
Study_guide_2010-01
... This is a list of topics we will be covering to help you in preparation for exams. Topics from Clayden are indicated clearly by chapter and page numbers where necessary. Topics NOT from Clayden are listed in italics. PLTL topics are in CAPS. This document will be updated throughout the term. The goa ...
... This is a list of topics we will be covering to help you in preparation for exams. Topics from Clayden are indicated clearly by chapter and page numbers where necessary. Topics NOT from Clayden are listed in italics. PLTL topics are in CAPS. This document will be updated throughout the term. The goa ...
Wine Country Lodging near San Luis Obispo CA
... as the EAN rule, that in a number of metal complexes the metal atom tends to surround itself with sufficient ligands that the resul�ng effec�ve atomic number is numerically equal to the atomic number ...
... as the EAN rule, that in a number of metal complexes the metal atom tends to surround itself with sufficient ligands that the resul�ng effec�ve atomic number is numerically equal to the atomic number ...
Lecture 22 Enzyme mechanisms - II
... facilitating by the abstraction of proton by His 57 from that water molecule. The process is followed by the leaving of Ser 195 which in turn results carboxylate product (the new Cterminal portion of the cleaved polypeptide chain). ...
... facilitating by the abstraction of proton by His 57 from that water molecule. The process is followed by the leaving of Ser 195 which in turn results carboxylate product (the new Cterminal portion of the cleaved polypeptide chain). ...
VIPEr_LO_Byers_PLA_PolymerizationCatalyst
... spin. Describe the Evans’ method and how it can be used to get information about the spin state of a metal center. What other technique could be used to obtain the same information? (You will need to use other sources to answer this questions.) 8. The article states that the iron has a distorted tri ...
... spin. Describe the Evans’ method and how it can be used to get information about the spin state of a metal center. What other technique could be used to obtain the same information? (You will need to use other sources to answer this questions.) 8. The article states that the iron has a distorted tri ...
Organic Chemistry 25.2 Introduction to Hydrocarbons
... Even though they contain ! bonds, aromatic hydrocarbons undergo substitution reactions more readily than addition reactions. ...
... Even though they contain ! bonds, aromatic hydrocarbons undergo substitution reactions more readily than addition reactions. ...
Study guide/lecture topics
... This is a list of topics we will be covering to help you in preparation for exams. Topics from Clayden are indicated clearly by chapter and page numbers where necessary. Topics NOT from Clayden are listed in italics. PLTL topics are in CAPS. This document will be updated throughout the term. The goa ...
... This is a list of topics we will be covering to help you in preparation for exams. Topics from Clayden are indicated clearly by chapter and page numbers where necessary. Topics NOT from Clayden are listed in italics. PLTL topics are in CAPS. This document will be updated throughout the term. The goa ...
Oxidation States of Ruthenium and Osmium
... A. K. Keep, Platinum Metals Rev., 2004, 48, (2), 64 J. M. Fisher, R. J. Potter and C. F. J. Barnard, Platinum Metals Rev., 2004, 48, (3), 101 ...
... A. K. Keep, Platinum Metals Rev., 2004, 48, (2), 64 J. M. Fisher, R. J. Potter and C. F. J. Barnard, Platinum Metals Rev., 2004, 48, (3), 101 ...
Functional Groups - La Salle University
... where R’ is different from R – These can be considered organic derivatives of water in which both hydrogens are replaced by organic groups – The bond angle at oxygen is close to the tetrahedral angle ...
... where R’ is different from R – These can be considered organic derivatives of water in which both hydrogens are replaced by organic groups – The bond angle at oxygen is close to the tetrahedral angle ...
Chapter 13: EDTA titrations
... Chapter 13: EDTA titrations Complexation Reaction: A reaction between two species having a well-defined stoichiometry. The resulting bond is not permanent from a covalent standpoint. Complex: The resulting structure formed during a complexation reaction. Coordination Center: Metal ion in a complex ( ...
... Chapter 13: EDTA titrations Complexation Reaction: A reaction between two species having a well-defined stoichiometry. The resulting bond is not permanent from a covalent standpoint. Complex: The resulting structure formed during a complexation reaction. Coordination Center: Metal ion in a complex ( ...
Mixed ligand transition metal(II) complexes of Knoevenagel
... ioni c bonding. The observed value of Cf? is 0.75-0.79. indicates that the co mplexes have covalent character. The out-of-plane n -bonding (y-) and in-plan e nbonding (~") parameters are also ca lcul ated from the following expressions: ...
... ioni c bonding. The observed value of Cf? is 0.75-0.79. indicates that the co mplexes have covalent character. The out-of-plane n -bonding (y-) and in-plan e nbonding (~") parameters are also ca lcul ated from the following expressions: ...
L11S08
... In aqueous solutions iron is present as the +2 (ferrous) or +3 (ferric) oxidation states. Iron reacts with non-oxidizing acids to form Fe2+(aq) In the presence of air, Fe2+ is oxidized to Fe3+. In acid solution, Fe(H2O)63+ forms, but in base Fe(OH)3 precipitates. ...
... In aqueous solutions iron is present as the +2 (ferrous) or +3 (ferric) oxidation states. Iron reacts with non-oxidizing acids to form Fe2+(aq) In the presence of air, Fe2+ is oxidized to Fe3+. In acid solution, Fe(H2O)63+ forms, but in base Fe(OH)3 precipitates. ...
File
... 2. Change the ending of the parent chain from e to one 3. Identify and number any substituent groups. Numbering starts at end closest to the C=O group. ...
... 2. Change the ending of the parent chain from e to one 3. Identify and number any substituent groups. Numbering starts at end closest to the C=O group. ...
Chapter_Sixteen_lecture
... 2. Ketones, do not have this hydrogen and do not react with mild oxidizing agents. ...
... 2. Ketones, do not have this hydrogen and do not react with mild oxidizing agents. ...
New Materials from Metal Vapour Chemistry
... The outcome of such a series of reactions is not simply controlled by the thermodynamics of the system. One must also take into account the relative rates of each step in the chain of events that lead to intermediates and products in the reaction grid of Scheme I. As many of the steps usually have z ...
... The outcome of such a series of reactions is not simply controlled by the thermodynamics of the system. One must also take into account the relative rates of each step in the chain of events that lead to intermediates and products in the reaction grid of Scheme I. As many of the steps usually have z ...
CHAPTER 11
... pairs forms coordinate bonds with metal ions. • Ligand – an ion or molecule that forms a covalent bond with a cation or a neutral metal atom by donating a pair of electrons that are then shared by the two. ...
... pairs forms coordinate bonds with metal ions. • Ligand – an ion or molecule that forms a covalent bond with a cation or a neutral metal atom by donating a pair of electrons that are then shared by the two. ...
p – Block Elements 1
... (iii) The Electronegativity values of group 13 > than group 1 & 2. When these elements react with other elements, the difference in electronegativity is small which favours the formation of covalent bond. In +3 oxidation state, the no. of electron around the central atom in a molecule of the compoun ...
... (iii) The Electronegativity values of group 13 > than group 1 & 2. When these elements react with other elements, the difference in electronegativity is small which favours the formation of covalent bond. In +3 oxidation state, the no. of electron around the central atom in a molecule of the compoun ...
Chemistry 3211 - Inorganic Chemistry of the Transition Metals
... oxidant instead of stoichiometric, toxic metal oxidants such as dichromate. Other catalytic research involves reactions in water or room temperature ionic liquids instead of volatile organic solvents. These techniques and others allow catalysts to be recycled more easily. New catalysts are being dev ...
... oxidant instead of stoichiometric, toxic metal oxidants such as dichromate. Other catalytic research involves reactions in water or room temperature ionic liquids instead of volatile organic solvents. These techniques and others allow catalysts to be recycled more easily. New catalysts are being dev ...
Nanocomposites based on nanosized inorganic particles and
... generally insoluble and infusible when more than two pyridine groups are attached to a metal salt. The co-ordination bond would crosslink the polymer, producing network structure. The formation of inter molecular co-ordination bonds is most prominent for p4vp where as poly(2 vinyl pyridine)[p2vp] is ...
... generally insoluble and infusible when more than two pyridine groups are attached to a metal salt. The co-ordination bond would crosslink the polymer, producing network structure. The formation of inter molecular co-ordination bonds is most prominent for p4vp where as poly(2 vinyl pyridine)[p2vp] is ...
Metal carbonyl

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel carbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometalic complexes.Metal carbonyls are toxic by skin contact, inhalation or ingestion, in part because of their ability to carbonylate hemoglobin to give carboxyhemoglobin, which prevents the binding of O2.























