Week #10: The Synthesis of a Compound using a Limiting Reagent
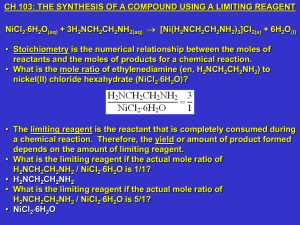
... • The limiting reagent is the reactant that is completely consumed during a chemical reaction. Therefore, the yield or amount of product formed depends on the amount of limiting reagent. • What is the limiting reagent if the actual mole ratio of H2NCH2CH2NH2 / NiCl26H2O is 1/1? • H2NCH2CH2NH2 • Wha ...
... • The limiting reagent is the reactant that is completely consumed during a chemical reaction. Therefore, the yield or amount of product formed depends on the amount of limiting reagent. • What is the limiting reagent if the actual mole ratio of H2NCH2CH2NH2 / NiCl26H2O is 1/1? • H2NCH2CH2NH2 • Wha ...
Document
... • The limiting reagent is the reactant that is completely consumed during a chemical reaction. Therefore, the yield or amount of product formed depends on the amount of limiting reagent. • What is the limiting reagent if the actual mole ratio of H2NCH2CH2NH2 / NiCl26H2O is 1/1? • H2NCH2CH2NH2 • Wha ...
... • The limiting reagent is the reactant that is completely consumed during a chemical reaction. Therefore, the yield or amount of product formed depends on the amount of limiting reagent. • What is the limiting reagent if the actual mole ratio of H2NCH2CH2NH2 / NiCl26H2O is 1/1? • H2NCH2CH2NH2 • Wha ...
Functional Group Handout
... E. Amides: contain a carbonyl group. The carbon atom of the carbonyl group is bonded to another carbon atom and a nitrogen atom. Amides can be primary secondary or tertiary. The nitrogen atom of the primary amide is bonded to the carbonyl carbon and two hydrogens. The nitrogen atom of a secondary am ...
... E. Amides: contain a carbonyl group. The carbon atom of the carbonyl group is bonded to another carbon atom and a nitrogen atom. Amides can be primary secondary or tertiary. The nitrogen atom of the primary amide is bonded to the carbonyl carbon and two hydrogens. The nitrogen atom of a secondary am ...
File
... When concentrated hydrochloric acid is added to a solution containing hydrated copper(II) ions, the colour of the solution changes from light blue to green. The equation for the reaction is: [Cu(H2O)6]2+(aq) + 4Cl–(aq) → [CuCl4]2–(aq) + 6H2O(l) ...
... When concentrated hydrochloric acid is added to a solution containing hydrated copper(II) ions, the colour of the solution changes from light blue to green. The equation for the reaction is: [Cu(H2O)6]2+(aq) + 4Cl–(aq) → [CuCl4]2–(aq) + 6H2O(l) ...
STABILITETEN AF KOORDINATIONSFORBINDELSER
... In this case it is assumed that the ligand in the medium used has no relevant acid base properties (i.e. is a very weak base). If the ligand is not a very weak base CL should be replaced by elaborate on that question when relevant. In a given solution with the total concentrations CM and CL an equil ...
... In this case it is assumed that the ligand in the medium used has no relevant acid base properties (i.e. is a very weak base). If the ligand is not a very weak base CL should be replaced by elaborate on that question when relevant. In a given solution with the total concentrations CM and CL an equil ...
Alfred Werner
... [Co(NH3)5Cl]Cl2 – [Co(NH3)5Cl]2+ and two Cl-; Co is in +3 oxidation state K3[Fe(CN)6] – [Fe(CN)6]3- and three K+; Fe is in +3 oxidation state Coordination number is the Number of bonds formed between the metal ion and the ligands in the complex ion. The most common coordination numbers are 4 and 6. ...
... [Co(NH3)5Cl]Cl2 – [Co(NH3)5Cl]2+ and two Cl-; Co is in +3 oxidation state K3[Fe(CN)6] – [Fe(CN)6]3- and three K+; Fe is in +3 oxidation state Coordination number is the Number of bonds formed between the metal ion and the ligands in the complex ion. The most common coordination numbers are 4 and 6. ...
Formation of rare earth metal complexes with Zonisamide in
... complexes with medicinal drugs plays a major role in the biological and chemical activity. Metal complexes of medicinal drugs have played a central role in the development of coordination chemistry. Metal complexes are widely used in various fields, such as biological processes, pharmaceuticals, sep ...
... complexes with medicinal drugs plays a major role in the biological and chemical activity. Metal complexes of medicinal drugs have played a central role in the development of coordination chemistry. Metal complexes are widely used in various fields, such as biological processes, pharmaceuticals, sep ...
A millennial overview of transition metal chemistry
... and the many chemical processes, including catalytic ones, in which they participate.11 Of course, it is not only metal carbonyls per se that are important but all of the many related low-valent complexes with ligands such as NO, phosphines, isocyanides, and so forth. The major indirect reason for t ...
... and the many chemical processes, including catalytic ones, in which they participate.11 Of course, it is not only metal carbonyls per se that are important but all of the many related low-valent complexes with ligands such as NO, phosphines, isocyanides, and so forth. The major indirect reason for t ...
PowerPoint - Organic Chemistry
... • All of these have a plant origin • All of these rely on the “fixing” of C from CO2 • Synthetic organic compounds are derived from fossil fuels or plant material ...
... • All of these have a plant origin • All of these rely on the “fixing” of C from CO2 • Synthetic organic compounds are derived from fossil fuels or plant material ...
(phenylalanine) schiff bases and their metal complexes
... acids containing uncharged amino groups, at physiological PH values, ay also undergo Schiff base formation, which presents another potential mechanism for metal complexes. Schiff bases are condensation products of primary amines with carbonyl compounds and they were first reported by Schiff in 1864. ...
... acids containing uncharged amino groups, at physiological PH values, ay also undergo Schiff base formation, which presents another potential mechanism for metal complexes. Schiff bases are condensation products of primary amines with carbonyl compounds and they were first reported by Schiff in 1864. ...
Anti microbial study of newly formed complexes of transition and
... spectrophotometer using KBr Pallets in the range of 4000 cm-1 to 400 cm-1 .Elemental analysis of C,H,N was performed on a carlo erba mode 1108 elemental analyzer.The Mass spectra were done on a jeol SX-102 spectrophotometer using argon as the FAB gas. Elico, SL191 double beam uv-vis spectrophotomete ...
... spectrophotometer using KBr Pallets in the range of 4000 cm-1 to 400 cm-1 .Elemental analysis of C,H,N was performed on a carlo erba mode 1108 elemental analyzer.The Mass spectra were done on a jeol SX-102 spectrophotometer using argon as the FAB gas. Elico, SL191 double beam uv-vis spectrophotomete ...
Transition-Metal Complexes with the Novel Scorpionate Ligand
... upon slow solvent evaporation as blue-violet crystals. Presumably, this recrystallization has been successful because of the availability of a stable metal-ammine complex in the form of tetraamminecopper. The crystallization of the copper complex is not without difficulties, however, since a white, ...
... upon slow solvent evaporation as blue-violet crystals. Presumably, this recrystallization has been successful because of the availability of a stable metal-ammine complex in the form of tetraamminecopper. The crystallization of the copper complex is not without difficulties, however, since a white, ...
Experiment title: Studies of cadmium and mercury ligand
... The EXAFS and XANES spectra were measured in fluorescence or transmission detection mode, depending on the concentration of the investigated element in the sample, at BM23 beamline. For Cd K-edge (26,711 eV) XAS a double crystal Si(311) monochromator with 2 eV resolution was used, while in the ener ...
... The EXAFS and XANES spectra were measured in fluorescence or transmission detection mode, depending on the concentration of the investigated element in the sample, at BM23 beamline. For Cd K-edge (26,711 eV) XAS a double crystal Si(311) monochromator with 2 eV resolution was used, while in the ener ...
Complexes of metal ions and nomenclature for inorganic compounds
... A complex is written such that everything inside the square brackets is a ligand chemically bonded to the metal ion. Everything outside the brackets is a counter-ion or something simply present in the crystal lattice. Thus, we might have [Co(H2O)6](NO3)2 where the NO3- ions are counter-ions. ...
... A complex is written such that everything inside the square brackets is a ligand chemically bonded to the metal ion. Everything outside the brackets is a counter-ion or something simply present in the crystal lattice. Thus, we might have [Co(H2O)6](NO3)2 where the NO3- ions are counter-ions. ...
Isomerism in octahedral metal complexesBJJ
... Isomerism in Octahedral Transition Metal Complexes As you have seen in the chemistry of carbon‐containing compounds, often there are several isomers possible for the same compound formula. Even when atoms are connected in the same order it is possible that we have not uniquely described the stru ...
... Isomerism in Octahedral Transition Metal Complexes As you have seen in the chemistry of carbon‐containing compounds, often there are several isomers possible for the same compound formula. Even when atoms are connected in the same order it is possible that we have not uniquely described the stru ...
Transition metal complexes_bonding
... You are probably wondering: Why are some complexes high-spin and others low spin? Can you predict it? Valence bond theory describes bonding in complexes consistent with observed magnetic properties; it cannot explain why the ligands dictate one over the other Enter the crystal field theory… ...
... You are probably wondering: Why are some complexes high-spin and others low spin? Can you predict it? Valence bond theory describes bonding in complexes consistent with observed magnetic properties; it cannot explain why the ligands dictate one over the other Enter the crystal field theory… ...
Sample 3 - University of Puget Sound
... within drug syntheses, efficient catalysis for these reactions will contribute significantly to decreasing the waste produced by the pharmaceutical industry and thereby promoting green chemistry. Current catalysts for such reactions are highly efficient but also toxic and expensive. Developing effic ...
... within drug syntheses, efficient catalysis for these reactions will contribute significantly to decreasing the waste produced by the pharmaceutical industry and thereby promoting green chemistry. Current catalysts for such reactions are highly efficient but also toxic and expensive. Developing effic ...
π bonded ligands (# 2)
... This allows the bonding p orbital on these carbons to point more directly toward the metal, thus further improving the M‐L overlap. ...
... This allows the bonding p orbital on these carbons to point more directly toward the metal, thus further improving the M‐L overlap. ...
Theoretical Competition - Austrian Chemistry Olympiad
... metals there is a set of problems which is connected with analytical and/or physical chemistry. Which metals are we talking about? In order to answer this question there is a series of information available: ...
... metals there is a set of problems which is connected with analytical and/or physical chemistry. Which metals are we talking about? In order to answer this question there is a series of information available: ...
Carbonyl Alpha-Substitution Reactions
... • Malonic ester (diethyl propanedioate) is easily converted into its enolate ion by reaction with sodium ethoxide in ethanol • The enolate is a good nucleophile that reacts rapidly with an alkyl halide to give an -substituted malonic ester ...
... • Malonic ester (diethyl propanedioate) is easily converted into its enolate ion by reaction with sodium ethoxide in ethanol • The enolate is a good nucleophile that reacts rapidly with an alkyl halide to give an -substituted malonic ester ...
IOSR Journal of Applied Chemistry (IOSR-JAC)
... 1.2 Synthesis of ligand Ligand HBBH is synthesized by the reported procedure 5. 1.3 Synthesis of metal complexes The metal complexes of Cu (II), Ni (II), Co (II) and Mn(II) were prepared by mixing ethanolic solution of the ligand (E)-N'-[2-hydroxy benzylidene] benzohydrazide (HBBH) and corresponding ...
... 1.2 Synthesis of ligand Ligand HBBH is synthesized by the reported procedure 5. 1.3 Synthesis of metal complexes The metal complexes of Cu (II), Ni (II), Co (II) and Mn(II) were prepared by mixing ethanolic solution of the ligand (E)-N'-[2-hydroxy benzylidene] benzohydrazide (HBBH) and corresponding ...
Aldehid dan Keton
... comparable alkane or ether. • Cannot H-bond to each other, so lower boiling point than comparable alcohol. ...
... comparable alkane or ether. • Cannot H-bond to each other, so lower boiling point than comparable alcohol. ...
Metal carbonyl

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel carbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometalic complexes.Metal carbonyls are toxic by skin contact, inhalation or ingestion, in part because of their ability to carbonylate hemoglobin to give carboxyhemoglobin, which prevents the binding of O2.