5 Complexation
... • A suitable buffer solution and indicator are added to the metal ion solution and the solution titrated with standard disodium edetat until the indicator just changes color. A blank titration may be performed, omitting the analyte as a check on the presence of traces of metallic impurities in the r ...
... • A suitable buffer solution and indicator are added to the metal ion solution and the solution titrated with standard disodium edetat until the indicator just changes color. A blank titration may be performed, omitting the analyte as a check on the presence of traces of metallic impurities in the r ...
Synthesis and Spectroscopy Study of the New Amide Complexes of
... The chemistry of metal complexes containing amide ligands has been a subject of great interest. The reason for this interest stems from the fact that such complexes can be easily made and variation of the substituents is facile (4). This is due to partly to the stabilization of the M-amido bond by π ...
... The chemistry of metal complexes containing amide ligands has been a subject of great interest. The reason for this interest stems from the fact that such complexes can be easily made and variation of the substituents is facile (4). This is due to partly to the stabilization of the M-amido bond by π ...
CARBONYL COMPOUNDS ALDEHYDES AND KETONES
... The most common oxidation reaction of carbonyl compounds is the oxidation of aldehydes to carboxylic acids. A variety of oxidizing agents can be used, including CrO3, Na2Cr2O7, K2Cr2O7 and KMnO4. Aldehydes are also oxidized selectively in the presence of other functional groups using silver(I) oxide ...
... The most common oxidation reaction of carbonyl compounds is the oxidation of aldehydes to carboxylic acids. A variety of oxidizing agents can be used, including CrO3, Na2Cr2O7, K2Cr2O7 and KMnO4. Aldehydes are also oxidized selectively in the presence of other functional groups using silver(I) oxide ...
CHM412 June 2013 paper
... alcohold that can be oxided to methyl carbonyls will also give a positive test result for this reaction, because the reagents used in the triiodomethane test are oxidising, i.e. I 2(aq) + NaOH. They oxidise methyl 1o alcohols and methyl 2o alcohols to methyl carbonyls, which then undergo the usual b ...
... alcohold that can be oxided to methyl carbonyls will also give a positive test result for this reaction, because the reagents used in the triiodomethane test are oxidising, i.e. I 2(aq) + NaOH. They oxidise methyl 1o alcohols and methyl 2o alcohols to methyl carbonyls, which then undergo the usual b ...
Chapter 7 7.4 Name and draw structures of the following complexes?
... putting the two bulky triphenylphosphine ligands opposite one another in the two trigonalbipyramidal complexes. The names of the three complexes, from left to right, are carbonyl( chloro)bis(triphenylphosphine)iridium(I), dicarbonyl(chloro)bis(triphenylphosphine)iridium(I), and dicarbonyl(hydrido)bi ...
... putting the two bulky triphenylphosphine ligands opposite one another in the two trigonalbipyramidal complexes. The names of the three complexes, from left to right, are carbonyl( chloro)bis(triphenylphosphine)iridium(I), dicarbonyl(chloro)bis(triphenylphosphine)iridium(I), and dicarbonyl(hydrido)bi ...
Carbohydrate-Metal Interactions Shaped by Supramolecular
... well-suited ligand in terms of the O5 pattern of its bIII III IV furanoside isomer, forms dinuclear FeIII 2 , Mn2 , and Mn Mn complexes in the neutral or alkaline pH region with two entirely deprotonated mannosefuranose anions as the only ligands.[1, 2] Deprotonated carbohydrate ligands are apparent ...
... well-suited ligand in terms of the O5 pattern of its bIII III IV furanoside isomer, forms dinuclear FeIII 2 , Mn2 , and Mn Mn complexes in the neutral or alkaline pH region with two entirely deprotonated mannosefuranose anions as the only ligands.[1, 2] Deprotonated carbohydrate ligands are apparent ...
壹 - 國立彰化師範大學圖書館
... 12. Explain how the laser generates light. What is meant by population inversion in laser action? (10 %) 13. What would be the effect of the following on the “plate height” of a column in gas chromatography? Explain! (6 %) (a) Increasing the weight of the stationary phase relative to the packing wei ...
... 12. Explain how the laser generates light. What is meant by population inversion in laser action? (10 %) 13. What would be the effect of the following on the “plate height” of a column in gas chromatography? Explain! (6 %) (a) Increasing the weight of the stationary phase relative to the packing wei ...
Transition Elements/Coordination Chemistry
... Oxidation States: Unlike main group metals, transition metals often exhibit multiple oxidation states Highest oxidation state is the same as the group number for Groups 3B to 7B ...
... Oxidation States: Unlike main group metals, transition metals often exhibit multiple oxidation states Highest oxidation state is the same as the group number for Groups 3B to 7B ...
Cu(NH3)4 - Granite Bay High School / Granite Bay High School
... (8) chelates are complexes formed by polydentate ligands; "chelate" comes from Greek for crab's claw (9) complexes used in sensitive qualitative tests to detect presence of ions in parts per million (ppm) range (10) hydrated ionic salts have water molecules as ligands and are thus complex ions/coord ...
... (8) chelates are complexes formed by polydentate ligands; "chelate" comes from Greek for crab's claw (9) complexes used in sensitive qualitative tests to detect presence of ions in parts per million (ppm) range (10) hydrated ionic salts have water molecules as ligands and are thus complex ions/coord ...
Transition Chemistry
... This difference occurs because the last electrons added to the transition metal elements are inner electrons: d electrons for the d-block transition metals and f electrons for the lanthanides and actinides. These inner d and f electrons cannot participate in bonding as readily as the valence s and p ...
... This difference occurs because the last electrons added to the transition metal elements are inner electrons: d electrons for the d-block transition metals and f electrons for the lanthanides and actinides. These inner d and f electrons cannot participate in bonding as readily as the valence s and p ...
Chiral-Auxiliary-Mediated Asymmetric Synthesis of Tris
... Next, we continued with the highly diastereopure complex ΛRuSC-7 and set out to synthesize Λ-1 by replacing the salicyloxazoline ligand with bpy with retention of configuration.8 In preliminary experiments we recognized the acid lability of the salicyloxazoline ligand in complexes of type ΛRu-SC-5-7 ...
... Next, we continued with the highly diastereopure complex ΛRuSC-7 and set out to synthesize Λ-1 by replacing the salicyloxazoline ligand with bpy with retention of configuration.8 In preliminary experiments we recognized the acid lability of the salicyloxazoline ligand in complexes of type ΛRu-SC-5-7 ...
Assignment 6
... 7. K3[Co(CN)6] is colorless while K3[Co(C2O4)] is colored. Why ? 8. Cr(H2O)6]2+ is dark pink whereas [Mn(H2O)6]2+ is pale pink. Explain 9. Which of the following ligands F-, CN-, H2O and NO2 will form significantly distorted octahedral complexes with cobalt (II) ? Explain. 10. Which of the following ...
... 7. K3[Co(CN)6] is colorless while K3[Co(C2O4)] is colored. Why ? 8. Cr(H2O)6]2+ is dark pink whereas [Mn(H2O)6]2+ is pale pink. Explain 9. Which of the following ligands F-, CN-, H2O and NO2 will form significantly distorted octahedral complexes with cobalt (II) ? Explain. 10. Which of the following ...
Stability Constants - World Chemistry Tutor
... Let’s take the equilibrium for the substitution of NH3 ligands by EDTA shown above. We can predict the entropy change for the reaction to be high. Remember that entropy is a measure of disorder – the more disorder, the higher the entropy. Reactions tend to favour an increase in entropy. This is not ...
... Let’s take the equilibrium for the substitution of NH3 ligands by EDTA shown above. We can predict the entropy change for the reaction to be high. Remember that entropy is a measure of disorder – the more disorder, the higher the entropy. Reactions tend to favour an increase in entropy. This is not ...
Metal Complexes and Isomerism 197. What is a coordination
... 221. Show that for the systems d1, d2, d3, d8, d9 and d10 two different spin states (high spin and low spin) are not possible. Show that for the systems d4-d7 two different spin states are possible dependent on the field strength of the ligand. 222. What type of electronic configurations for metal c ...
... 221. Show that for the systems d1, d2, d3, d8, d9 and d10 two different spin states (high spin and low spin) are not possible. Show that for the systems d4-d7 two different spin states are possible dependent on the field strength of the ligand. 222. What type of electronic configurations for metal c ...
CHE450G Final Exam CP-109 December 11, 2006 10:30
... π-acceptor (π-acids): ligands that do have empty π symmetry orbitals (p or π*) that can engage in π-bonding with transition metal orbitals (t2g set in Oh complexes) and no filled π symmetry orbitals that are close in energy to the metal orbitals; increase Δo. (e.g. CO) (b) Use a partial molecular or ...
... π-acceptor (π-acids): ligands that do have empty π symmetry orbitals (p or π*) that can engage in π-bonding with transition metal orbitals (t2g set in Oh complexes) and no filled π symmetry orbitals that are close in energy to the metal orbitals; increase Δo. (e.g. CO) (b) Use a partial molecular or ...
Document
... to aldhydes, yielding allylic or homoallylic alcohols. The stereochemical unpredictability of these allyl and vinyl organometallics often limit their use in natural products synthesis, restricting their role to early synthetic steps where functionality is typically unlimited. The Nozaki-Hiyama react ...
... to aldhydes, yielding allylic or homoallylic alcohols. The stereochemical unpredictability of these allyl and vinyl organometallics often limit their use in natural products synthesis, restricting their role to early synthetic steps where functionality is typically unlimited. The Nozaki-Hiyama react ...
Chem 4471 - Transition Metals and Catalysis
... Advanced Inorganic Chemistry, by Cotton and Wilkinson et al., Wiley. There are also several excellent texts on organometallic chemistry and catalysis which are recommended, including: Organometallics, by C. Elschenbroich and A. Salzer, VCH. The Organometallic Chemistry of the Transition Metals, by R ...
... Advanced Inorganic Chemistry, by Cotton and Wilkinson et al., Wiley. There are also several excellent texts on organometallic chemistry and catalysis which are recommended, including: Organometallics, by C. Elschenbroich and A. Salzer, VCH. The Organometallic Chemistry of the Transition Metals, by R ...
Complexometric Titrations
... replacement of one or more of the coordinated solvent molecules by other Nucleophilic groups. The groups bound to the central ion are called ligands and in aqueous solution the reaction can be represented by the equation: Mm+(H2O)n + L [Mm+(H2O)(n-1)L] + H2O ...
... replacement of one or more of the coordinated solvent molecules by other Nucleophilic groups. The groups bound to the central ion are called ligands and in aqueous solution the reaction can be represented by the equation: Mm+(H2O)n + L [Mm+(H2O)(n-1)L] + H2O ...
Reactions of Carbonyl compounds
... 2,4-DINITROPHENYLHYDRAZINE C6H3(NO2)2NHNH2 The following structural isomers have similar boiling points because of similar van der Waals forces and dipole-dipole interactions. They would be impossible to identify with any precision using boiling point determination. ...
... 2,4-DINITROPHENYLHYDRAZINE C6H3(NO2)2NHNH2 The following structural isomers have similar boiling points because of similar van der Waals forces and dipole-dipole interactions. They would be impossible to identify with any precision using boiling point determination. ...
Get Day 17 - Mattson Creighton
... Inorganic Chemistry with Doc M. Day 17. Transition Metals Complexes III: Ligand Field Theory (MO Theory) Topics: 1. Molecular orbital theory and the octahedron 2. The MO energy diagram and Δo 1. Octahedral transition metal complexes utilize s, p and d-orbitals in their bonding. For a first row trans ...
... Inorganic Chemistry with Doc M. Day 17. Transition Metals Complexes III: Ligand Field Theory (MO Theory) Topics: 1. Molecular orbital theory and the octahedron 2. The MO energy diagram and Δo 1. Octahedral transition metal complexes utilize s, p and d-orbitals in their bonding. For a first row trans ...
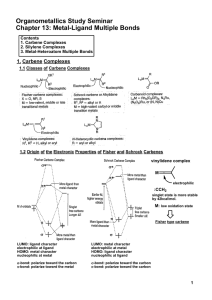
Metal-Ligand Multiple Bonds
... In general, multiple bonds to silicon and other second-row main group elements are less stable than multiple bonds to carbon, oxygen and nitrogen. ex) Si=O, M=Si: unstable ...
... In general, multiple bonds to silicon and other second-row main group elements are less stable than multiple bonds to carbon, oxygen and nitrogen. ex) Si=O, M=Si: unstable ...
THE COLORS OF COMPLEX METAL IONS
... For simplicity we are going to look at the octahedral complexes which have six simple (monodentate) ligands arranged around the central metal ion. The argument isn't really any different if you have multidentate ligands - it's just slightly more difficult to imagine! (WE only work with monodentate l ...
... For simplicity we are going to look at the octahedral complexes which have six simple (monodentate) ligands arranged around the central metal ion. The argument isn't really any different if you have multidentate ligands - it's just slightly more difficult to imagine! (WE only work with monodentate l ...
Handout-9
... In this case the alkyl anion is the best donor ligand compared to the more electronegative and poorly donating Cl anion. Note that the alkyl ligand (-CH2CH=CH2) initially coordinated to the Re after the oxidative addition is an η1-allyl ligand and that it can convert to the generally more stable η3 ...
... In this case the alkyl anion is the best donor ligand compared to the more electronegative and poorly donating Cl anion. Note that the alkyl ligand (-CH2CH=CH2) initially coordinated to the Re after the oxidative addition is an η1-allyl ligand and that it can convert to the generally more stable η3 ...
Metal carbonyl

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel carbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometalic complexes.Metal carbonyls are toxic by skin contact, inhalation or ingestion, in part because of their ability to carbonylate hemoglobin to give carboxyhemoglobin, which prevents the binding of O2.