S3 Chemistry - eduBuzz.org
... State whether covalent substances form discrete molecular or giant network structures Predict the typical properties of discrete molecular and giant network covalent structures State that ionic substances form giant lattices of oppositely charged ions and predict the typical properties of ioni ...
... State whether covalent substances form discrete molecular or giant network structures Predict the typical properties of discrete molecular and giant network covalent structures State that ionic substances form giant lattices of oppositely charged ions and predict the typical properties of ioni ...
Learning Outcomes for Chemical Reactions and
... • State whether covalent substances form discrete molecular or giant network structures • Predict the typical properties of discrete molecular and giant network covalent structures • State that ionic substances form giant lattices of oppositely charged ions and predict the typical properties of ioni ...
... • State whether covalent substances form discrete molecular or giant network structures • Predict the typical properties of discrete molecular and giant network covalent structures • State that ionic substances form giant lattices of oppositely charged ions and predict the typical properties of ioni ...
S3 Chemistry - eduBuzz.org
... State whether covalent substances form discrete molecular or giant network structures Predict the typical properties of discrete molecular and giant network covalent structures State that ionic substances form giant lattices of oppositely charged ions and predict the typical properties of ioni ...
... State whether covalent substances form discrete molecular or giant network structures Predict the typical properties of discrete molecular and giant network covalent structures State that ionic substances form giant lattices of oppositely charged ions and predict the typical properties of ioni ...
syllabus for screening test (mcq type)
... Bonding in organic molecules : σ and π bonds, bond distance, bond angle, and bond energy. Dipole moment of organic molecules. Inductive, resonance and hyperconjugative effect. Hydrogen bond. Tautomerism, Aromaticity, Huckel’s rule, aromatic, non aromatic and anti aromatic compounds. Effects of struc ...
... Bonding in organic molecules : σ and π bonds, bond distance, bond angle, and bond energy. Dipole moment of organic molecules. Inductive, resonance and hyperconjugative effect. Hydrogen bond. Tautomerism, Aromaticity, Huckel’s rule, aromatic, non aromatic and anti aromatic compounds. Effects of struc ...
PowerPoint
... methane, with water as a byproduct. The water that is produced can then react with CO in the water-gas shift reaction, equation (2). In addition, both CO and methane can decompose to form carbon as in equations (3) and (4). ...
... methane, with water as a byproduct. The water that is produced can then react with CO in the water-gas shift reaction, equation (2). In addition, both CO and methane can decompose to form carbon as in equations (3) and (4). ...
Chemical Reactions
... ___________________. A chemical change may be observed during a chemical reaction when the following happens: (use figure 2.2a-e) 1. ________________________________________________ 2. ________________________________________________ 3. ________________________________________________ 4. ___________ ...
... ___________________. A chemical change may be observed during a chemical reaction when the following happens: (use figure 2.2a-e) 1. ________________________________________________ 2. ________________________________________________ 3. ________________________________________________ 4. ___________ ...
•What makes up an atom? Draw an atom
... • Compound behave differently than individual elements • EX. Na vs NaCl ...
... • Compound behave differently than individual elements • EX. Na vs NaCl ...
classification of chemical reactions
... Factors that affect the rates of Reactions Temperature: an ___________________in temperature _____________________ the rate of chemical reactions (particles move faster, so reaction rate increases) ...
... Factors that affect the rates of Reactions Temperature: an ___________________in temperature _____________________ the rate of chemical reactions (particles move faster, so reaction rate increases) ...
A.P. Chemistry
... Calculations using Titration Data: M1V1 = M2V2 M = mol/L Volume in liters (be sure to convert mL L) (p. 145-147) Problem: What volume of 16 M sulfuric acid must be used to prepare 1.5 L of a 0.10 M H2SO4 solution? ...
... Calculations using Titration Data: M1V1 = M2V2 M = mol/L Volume in liters (be sure to convert mL L) (p. 145-147) Problem: What volume of 16 M sulfuric acid must be used to prepare 1.5 L of a 0.10 M H2SO4 solution? ...
Chemical Synthesis Using Earth-Abundant Metal
... catalysis is used for a wide variety of reactions, we chose to target a single chemical reaction – forming a bond between a carbon and silicon atom – to frame our studies. Currently, catalytic methods for C-Si bond formation require Rh, Ir, or Pt catalysts, high temperatures (up to 200�C), and sacri ...
... catalysis is used for a wide variety of reactions, we chose to target a single chemical reaction – forming a bond between a carbon and silicon atom – to frame our studies. Currently, catalytic methods for C-Si bond formation require Rh, Ir, or Pt catalysts, high temperatures (up to 200�C), and sacri ...
Reactions Homework Packet
... no reaction, write NO REACTION. For the following assume all compounds are aqueous (dissolved in water). ...
... no reaction, write NO REACTION. For the following assume all compounds are aqueous (dissolved in water). ...
Year 9 Homework Task 9E-5 Reactions 5-7
... Task: Complete the diagrams. Describe, in words, what happens at each stage of the reaction. Explain what happens during the reaction, using particle diagrams. ...
... Task: Complete the diagrams. Describe, in words, what happens at each stage of the reaction. Explain what happens during the reaction, using particle diagrams. ...
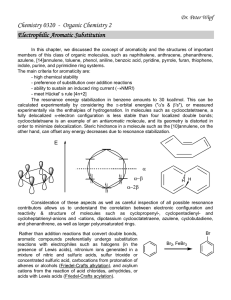
In this chapter, alkanes, alkenes, alkynes
... azulene, [14]annulene, toluene, phenol, aniline, benzoic acid, pyridine, pyrrole, furan, thiophene, indole, purine, and pyrimidine ring systems. The main criteria for aromaticity are: - high chemical stability - preference of substitution over addition reactions - ability to sustain an induced ring ...
... azulene, [14]annulene, toluene, phenol, aniline, benzoic acid, pyridine, pyrrole, furan, thiophene, indole, purine, and pyrimidine ring systems. The main criteria for aromaticity are: - high chemical stability - preference of substitution over addition reactions - ability to sustain an induced ring ...
water, h2o
... remaining ion rearranges to yield a Zundel ion, H5+O2. The excess proton fluctuates along the “proton coordinate”, between the two oxygen atoms and is trapped at either one as a new hydrogen bond (here, from a to b) reforms an Eigen ion – in this case on oxygen c. ...
... remaining ion rearranges to yield a Zundel ion, H5+O2. The excess proton fluctuates along the “proton coordinate”, between the two oxygen atoms and is trapped at either one as a new hydrogen bond (here, from a to b) reforms an Eigen ion – in this case on oxygen c. ...
Chapter 2 - Speedway High School
... • An anion is a negatively charged ion • A cation is a positively charged ion • An ionic bond is an attraction between an anion and a cation ...
... • An anion is a negatively charged ion • A cation is a positively charged ion • An ionic bond is an attraction between an anion and a cation ...
Chemical reactions
... For the hydrochloric acid we chose the coefficient 2 so that the number of chlorine atoms, as well as the number of ...
... For the hydrochloric acid we chose the coefficient 2 so that the number of chlorine atoms, as well as the number of ...
Chapter 14, Section 1, pages 494-501
... A chemical equilibrium is a state of balance between the forward and reverse reactions. The concentration of products and reactants remains unchanged. H2 + I2 <--------------> 2HI See fig. 3, pg. 498 Chemical Equilibria Are Dynamic (Leaky boat) Demo-pg. 500 Static equilibrium is a state when nothing ...
... A chemical equilibrium is a state of balance between the forward and reverse reactions. The concentration of products and reactants remains unchanged. H2 + I2 <--------------> 2HI See fig. 3, pg. 498 Chemical Equilibria Are Dynamic (Leaky boat) Demo-pg. 500 Static equilibrium is a state when nothing ...
Chemical Reactions
... The reactants are separated from each other by a plus sign and the products are separated from each other by a plus sign. There should be an arrow in the middle. Examples: When sodium is mixed with water, a purple alkaline solution of sodium hydroxide is produced and hydrogen gas is evolved. Sodium ...
... The reactants are separated from each other by a plus sign and the products are separated from each other by a plus sign. There should be an arrow in the middle. Examples: When sodium is mixed with water, a purple alkaline solution of sodium hydroxide is produced and hydrogen gas is evolved. Sodium ...
Chemical Reactions
... • We use chemical equations to summarize the process of the reactions • Chemical equations should be balanced in order to show that mass is conserved during a reaction • The principle that during chemical reactions, the mass of the products is always equal to the mass of the reactants, is known as t ...
... • We use chemical equations to summarize the process of the reactions • Chemical equations should be balanced in order to show that mass is conserved during a reaction • The principle that during chemical reactions, the mass of the products is always equal to the mass of the reactants, is known as t ...
Learning Standards vocab chemical basis and molecules of life 09
... Given the number of protons, identify the element using a Periodic Table. Explain the arrangement of the elements on the Periodic Table, including the significant relationships among elements in a given column or row. Explain how ions and ionic bonds are formed (e.g., sodium atoms lose an elec ...
... Given the number of protons, identify the element using a Periodic Table. Explain the arrangement of the elements on the Periodic Table, including the significant relationships among elements in a given column or row. Explain how ions and ionic bonds are formed (e.g., sodium atoms lose an elec ...
Exam practice answers 5
... (d) There are two equally viable ways of doing this: using a Hess’s triangle or a Born-Haber type cycle. Hess’s triangle method: ...
... (d) There are two equally viable ways of doing this: using a Hess’s triangle or a Born-Haber type cycle. Hess’s triangle method: ...
Chemical Equations and Reactions
... The negative ion of one compound replaces the negative ion of the other compound to form 2 new compounds. Usually forms a precipitate, water or a gas. ...
... The negative ion of one compound replaces the negative ion of the other compound to form 2 new compounds. Usually forms a precipitate, water or a gas. ...
Chapter 5 – Chemical Reactions
... Add a catalyst – a catalyst is a chemical that speeds up a reaction but does not get used up by the reaction ...
... Add a catalyst – a catalyst is a chemical that speeds up a reaction but does not get used up by the reaction ...
Cluster Fragmentation and Catalysis
... 1400 K) resolved the discrepancies between photochemical studies and high-temperature thermal studies. The large iodine anion becomes hydrophobic in relatively small water clusters because it disrupts the hydrogen bonding network. Therefore, for I-(H2O)64, the most stable structure occurs when the a ...
... 1400 K) resolved the discrepancies between photochemical studies and high-temperature thermal studies. The large iodine anion becomes hydrophobic in relatively small water clusters because it disrupts the hydrogen bonding network. Therefore, for I-(H2O)64, the most stable structure occurs when the a ...