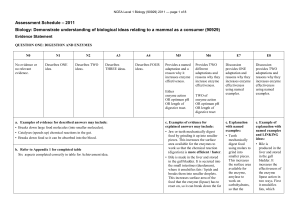
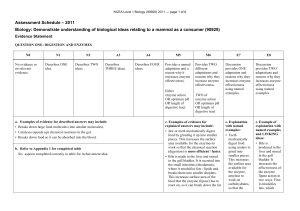
The Amino Acid and Carbohydrate Composition of the
... dissociation or tryptic digestion of virus particles and the amino acid and carbohydrate compositions of the two preparations are reported. The results indicate that the carbohydrate side-chains of the neuraminidase contain only N-acetylglucosamine, galactose, mannose and fucose, that they are attac ...
... dissociation or tryptic digestion of virus particles and the amino acid and carbohydrate compositions of the two preparations are reported. The results indicate that the carbohydrate side-chains of the neuraminidase contain only N-acetylglucosamine, galactose, mannose and fucose, that they are attac ...
Eukaryotic mRNA translation: Ribosome structure, function, and
... mRNP remodeling occurs during nucleocytoplasmic transport ...
... mRNP remodeling occurs during nucleocytoplasmic transport ...
P-glycoprotein Activation Monitored via ATP Hydrolysis and ATP
... We investigated the relationship between the rate of ATP hydrolysis and ATP synthesis upon P-glycoprotein activation for several structurally different drugs, including local anaesthetics, cyclic peptides, and cytotoxic drugs. ATP hydrolysis was assessed by spectroscopically monitoring the release o ...
... We investigated the relationship between the rate of ATP hydrolysis and ATP synthesis upon P-glycoprotein activation for several structurally different drugs, including local anaesthetics, cyclic peptides, and cytotoxic drugs. ATP hydrolysis was assessed by spectroscopically monitoring the release o ...
Mongar Higher Secondary School
... vi) Give reasons for the following. i) Table salt (NaCl) becomes moist and sticky during rainy season. ii) Alkalis should not be left exposed to air iii) Ionic compounds are bad conductors in solid state but are good conductiors in molten state or in their aqueous solutions. iv) The atomic size decr ...
... vi) Give reasons for the following. i) Table salt (NaCl) becomes moist and sticky during rainy season. ii) Alkalis should not be left exposed to air iii) Ionic compounds are bad conductors in solid state but are good conductiors in molten state or in their aqueous solutions. iv) The atomic size decr ...
CRICK: THE GENETIC CODE IS READ THREE BASES AT A TIME
... molecules into specific protein amino acid sequences, and quite another to understand how the trick is carried off. Is there one-to-one correspondence between a DNA base, an RNA base, and an amino acid? Clearly not, as there are 20 amino acids and only four types of nucleotide bases. A code of some ...
... molecules into specific protein amino acid sequences, and quite another to understand how the trick is carried off. Is there one-to-one correspondence between a DNA base, an RNA base, and an amino acid? Clearly not, as there are 20 amino acids and only four types of nucleotide bases. A code of some ...
Cellular Respiration
... • The inner membrane contains complexes of 5 integral membrane proteins that form the electron transport chain • The matrix contains a mixture of soluble enzymes that catalyze the breakdown of pyruvate. This series of enzymatic reactions is the Kreb's cycle. ...
... • The inner membrane contains complexes of 5 integral membrane proteins that form the electron transport chain • The matrix contains a mixture of soluble enzymes that catalyze the breakdown of pyruvate. This series of enzymatic reactions is the Kreb's cycle. ...
Proteins
... • Membrane proteins have more hydrophobic residues, whereas fibrous proteins may have atypical sequences • Homologous proteins from different organisms have homologous sequences • e.g., cytochrome c is highly conserved ...
... • Membrane proteins have more hydrophobic residues, whereas fibrous proteins may have atypical sequences • Homologous proteins from different organisms have homologous sequences • e.g., cytochrome c is highly conserved ...
Database Modeling in Bioinformatics
... • Full assessment and comparison not yet done • Manual annotation is best -especially if Medline number attached (biochemical evidence) • InterPro good, assuming protein hit is true and should hit all signatures in an entry • EC numbers good, but need mapping of protein to these, so may be extra ste ...
... • Full assessment and comparison not yet done • Manual annotation is best -especially if Medline number attached (biochemical evidence) • InterPro good, assuming protein hit is true and should hit all signatures in an entry • EC numbers good, but need mapping of protein to these, so may be extra ste ...
Welcome to Class 14 - (canvas.brown.edu).
... Net loss of NH3 from amino acids requires oxidation:! α-keto acids are 2 equivalents more oxidized than amino acids! ...
... Net loss of NH3 from amino acids requires oxidation:! α-keto acids are 2 equivalents more oxidized than amino acids! ...
Bio slides on cells - proteinsynthesis1unity
... •Packages these substances in vesicles for secretion out of cell •(Golgi vesicles) Cell secretions-eg: hormones, neurotransmitters(the brain chemicals that communicate information throughout our brain and body) are packaged in secretory vesicles by the Golgi body •The secretory vesicles are then tra ...
... •Packages these substances in vesicles for secretion out of cell •(Golgi vesicles) Cell secretions-eg: hormones, neurotransmitters(the brain chemicals that communicate information throughout our brain and body) are packaged in secretory vesicles by the Golgi body •The secretory vesicles are then tra ...
Ligand Binding - Stroud -Lecture 1
... • Thermodynamics of Protein Assembly • Structural Change on complexation • Empirical fitting of Atomic Interactions with Free Energy of Association • Estimate of free energy of H bonds and charge interactions in protein complexes and role of hydrophobic effect _______________________________________ ...
... • Thermodynamics of Protein Assembly • Structural Change on complexation • Empirical fitting of Atomic Interactions with Free Energy of Association • Estimate of free energy of H bonds and charge interactions in protein complexes and role of hydrophobic effect _______________________________________ ...
8.P.1.1Homework for Website
... 12. How many atoms of oxygen are in a molecule of glucose, C6H12O6? A. 24 B. 12 C. 6 D. 1 13. Which BEST explains how atoms combine to form compounds? A. The atoms in the compound share electrons B. The atoms in the compound share neutrons C. The atoms in the compound share protons 14. The compound ...
... 12. How many atoms of oxygen are in a molecule of glucose, C6H12O6? A. 24 B. 12 C. 6 D. 1 13. Which BEST explains how atoms combine to form compounds? A. The atoms in the compound share electrons B. The atoms in the compound share neutrons C. The atoms in the compound share protons 14. The compound ...
93KB - NZQA
... • Enzymes named: stating what they breakdown and what is produced. E.g. amylase breaks down starch into glucose ...
... • Enzymes named: stating what they breakdown and what is produced. E.g. amylase breaks down starch into glucose ...
LIPIDS - Biochemistry Notes
... DIGESTIVE MECHANISM FOR LIPIDS The average lipid intake is about 80g/day, of which more than 90% is triacylglycerol (TAG); the remainder consists of cholesterol, cholesteryl esters, phospholipids, free fatty acids 1. In the stomach: ...
... DIGESTIVE MECHANISM FOR LIPIDS The average lipid intake is about 80g/day, of which more than 90% is triacylglycerol (TAG); the remainder consists of cholesterol, cholesteryl esters, phospholipids, free fatty acids 1. In the stomach: ...
document
... Hydrogen bonds break, forming bubbles Enzymes unwind and unzip Free nucleotides in the nucleus start process of complementary base pairing Nucleotides are fused together by DNA ...
... Hydrogen bonds break, forming bubbles Enzymes unwind and unzip Free nucleotides in the nucleus start process of complementary base pairing Nucleotides are fused together by DNA ...
Biochemistry
_and_Carl_Ferdinand_Cori.jpg?width=300)
Biochemistry, sometimes called biological chemistry, is the study of chemical processes within and relating to living organisms. By controlling information flow through biochemical signaling and the flow of chemical energy through metabolism, biochemical processes give rise to the complexity of life. Over the last decades of the 20th century, biochemistry has become so successful at explaining living processes that now almost all areas of the life sciences from botany to medicine to genetics are engaged in biochemical research. Today, the main focus of pure biochemistry is in understanding how biological molecules give rise to the processes that occur within living cells, which in turn relates greatly to the study and understanding of whole organisms.Biochemistry is closely related to molecular biology, the study of the molecular mechanisms by which genetic information encoded in DNA is able to result in the processes of life. Depending on the exact definition of the terms used, molecular biology can be thought of as a branch of biochemistry, or biochemistry as a tool with which to investigate and study molecular biology.Much of biochemistry deals with the structures, functions and interactions of biological macromolecules, such as proteins, nucleic acids, carbohydrates and lipids, which provide the structure of cells and perform many of the functions associated with life. The chemistry of the cell also depends on the reactions of smaller molecules and ions. These can be inorganic, for example water and metal ions, or organic, for example the amino acids which are used to synthesize proteins. The mechanisms by which cells harness energy from their environment via chemical reactions are known as metabolism. The findings of biochemistry are applied primarily in medicine, nutrition, and agriculture. In medicine, biochemists investigate the causes and cures of disease. In nutrition, they study how to maintain health and study the effects of nutritional deficiencies. In agriculture, biochemists investigate soil and fertilizers, and try to discover ways to improve crop cultivation, crop storage and pest control.