Holt Chemistry – Guided Notes, Chapter 1
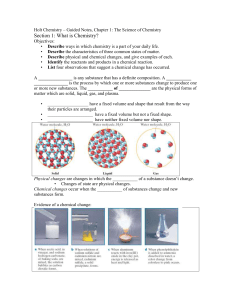
... A _______________ is any substance that has a definite composition. A ___________ _______________ is the process by which one or more substances change to produce one or more new substances. The ___________ of ______________ are the physical forms of matter which are solid, liquid, gas, and plasma. ...
... A _______________ is any substance that has a definite composition. A ___________ _______________ is the process by which one or more substances change to produce one or more new substances. The ___________ of ______________ are the physical forms of matter which are solid, liquid, gas, and plasma. ...
Day 5 Intro-to-Chem
... S Matter can be broken down into substances and mixtures. S Substances are pure. S Elements (smallest part of an element is an atom) S Compounds (smallest part of a compound is a molecule) S Mixtures of substances. NOT bonded together. S Mixtures are either homogeneous or heterogenous. ...
... S Matter can be broken down into substances and mixtures. S Substances are pure. S Elements (smallest part of an element is an atom) S Compounds (smallest part of a compound is a molecule) S Mixtures of substances. NOT bonded together. S Mixtures are either homogeneous or heterogenous. ...
lecture_CH1-2review_chem121pikul
... compounds rather then pure elements • Many are gases, some are solids at room temp, only Br2 is a liquid. ...
... compounds rather then pure elements • Many are gases, some are solids at room temp, only Br2 is a liquid. ...
MP 2 workbook 2016
... homogenous solution of wax, salt and iron. My hypothesis is that since this solution is a compound, it can be separated. I accomplished this by making a homogeneous compound of 5.0 grams of wax, 5.0 grams of copper salt and pinch of iron. In order to separate, the iron was first removed using filtra ...
... homogenous solution of wax, salt and iron. My hypothesis is that since this solution is a compound, it can be separated. I accomplished this by making a homogeneous compound of 5.0 grams of wax, 5.0 grams of copper salt and pinch of iron. In order to separate, the iron was first removed using filtra ...
Communicating Research to the General Public
... One of the major themes of inorganic chemistry is the study of metals, in particular transition metals. Transition metals, highlighted in red in Figure 1.1 comprise a majority of the periodic table and can be considered the building blocks of inorganic chemistry just as carbon is considered the buil ...
... One of the major themes of inorganic chemistry is the study of metals, in particular transition metals. Transition metals, highlighted in red in Figure 1.1 comprise a majority of the periodic table and can be considered the building blocks of inorganic chemistry just as carbon is considered the buil ...
File
... The three phases of matter (solids, liquids, and gases) have different properties. (3.1kk) A pure substance (element or compound) has a constant composition and constant properties throughout a given sample, and from sample to sample. (3.1r) Elements cannot be broken down by chemical change. (3.1u) ...
... The three phases of matter (solids, liquids, and gases) have different properties. (3.1kk) A pure substance (element or compound) has a constant composition and constant properties throughout a given sample, and from sample to sample. (3.1r) Elements cannot be broken down by chemical change. (3.1u) ...
+ O2 (g)
... Methane gas reacts with oxygen gas to produce carbon dioxide gas and gaseous water.! CH4(g) + O2(g) ➜ CO2(g) + H2O(g) This equation reads “1 molecule of CH4 gas combines with 1 molecule of O2 gas to make 1 molecule of CO2 gas and 1 molecule of H2O gas.” ...
... Methane gas reacts with oxygen gas to produce carbon dioxide gas and gaseous water.! CH4(g) + O2(g) ➜ CO2(g) + H2O(g) This equation reads “1 molecule of CH4 gas combines with 1 molecule of O2 gas to make 1 molecule of CO2 gas and 1 molecule of H2O gas.” ...
Name
... Ⓡ 8.5 (D) Chemical Formulas: Students will be able to recognize that chemical formulas are used to identify substances and determine the number of atoms of each element. Ⓡ 8.5 (E) Chemical Reactions: Students will be able to investigate how evidences of chemical reactions indicate that new substance ...
... Ⓡ 8.5 (D) Chemical Formulas: Students will be able to recognize that chemical formulas are used to identify substances and determine the number of atoms of each element. Ⓡ 8.5 (E) Chemical Reactions: Students will be able to investigate how evidences of chemical reactions indicate that new substance ...
What has been presented?
... Conductors: materials that conduct electricity. A current will be generated when a potential is applied to a material Ohm’s Law – V = IR Resistivity - resistance dependent on material properties resistivity - ρ of a material: resistance (R) per unit length (L)and cross-sectional area (A) r = RA/L Co ...
... Conductors: materials that conduct electricity. A current will be generated when a potential is applied to a material Ohm’s Law – V = IR Resistivity - resistance dependent on material properties resistivity - ρ of a material: resistance (R) per unit length (L)and cross-sectional area (A) r = RA/L Co ...
Notes
... • Physical property - is observed without changing the composition or identity of a substance • Physical change - produces a recognizable difference in the appearance of a substance without causing any change in its composition or identity - conversion from one physical state to another - melting an ...
... • Physical property - is observed without changing the composition or identity of a substance • Physical change - produces a recognizable difference in the appearance of a substance without causing any change in its composition or identity - conversion from one physical state to another - melting an ...
High School Curriculum Standards: Chemistry
... 2000 years old, but the idea of using properties of these particles to explain observable characteristics of matter has more recent origins. In ancient Greece, it was proposed that matter is composed of particles of four elements (earth, air, water, and fire) and that these particles are in continua ...
... 2000 years old, but the idea of using properties of these particles to explain observable characteristics of matter has more recent origins. In ancient Greece, it was proposed that matter is composed of particles of four elements (earth, air, water, and fire) and that these particles are in continua ...
Chemistry Vocab for Quiz 12/21 or 12/22 Atom – The smallest
... Element – A substance that cannot be broken down into another substance by physical or chemical means. Compound – A substance made of 2 or elements chemically combined in a specific ratio. Chemical bond – the force that holds 2 atoms together. Mixture – Two or more substances that are mixed together ...
... Element – A substance that cannot be broken down into another substance by physical or chemical means. Compound – A substance made of 2 or elements chemically combined in a specific ratio. Chemical bond – the force that holds 2 atoms together. Mixture – Two or more substances that are mixed together ...
Second Semester Extra Review
... c) heat 4. What factors determine whether a reaction is spontaneous or not? 5. Calculate the Gibb’s free energy if the entropy is 0.555 kJ/mol K and enthalpy is 56.9 kJ/mol at 25C. Is this reaction spontaneous? 6. What factors affect rate of a reaction? 7. What are the two conditions to have an eff ...
... c) heat 4. What factors determine whether a reaction is spontaneous or not? 5. Calculate the Gibb’s free energy if the entropy is 0.555 kJ/mol K and enthalpy is 56.9 kJ/mol at 25C. Is this reaction spontaneous? 6. What factors affect rate of a reaction? 7. What are the two conditions to have an eff ...
Ionic compound
... Solubility Following the aphorism, "like dissolves like", ionic compounds dissolve in polar solvents, especially those that ionize, such as water and ionic liquids. They are usually appreciably soluble in other polar solvents such as alcohols, acetone and dimethyl sulfoxide as well. Ionic compounds ...
... Solubility Following the aphorism, "like dissolves like", ionic compounds dissolve in polar solvents, especially those that ionize, such as water and ionic liquids. They are usually appreciably soluble in other polar solvents such as alcohols, acetone and dimethyl sulfoxide as well. Ionic compounds ...
AP Chemistry 2013 Semester 1 Final Exam Review Problems
... atomic subshell energies and electron assignments; atomic electron configurations; electron configurations of ions; atomic properties and periodic trends; periodic trends and chemical properties. 12. An electron microscope employs a beam of electrons to obtain an image of an object. What energy must ...
... atomic subshell energies and electron assignments; atomic electron configurations; electron configurations of ions; atomic properties and periodic trends; periodic trends and chemical properties. 12. An electron microscope employs a beam of electrons to obtain an image of an object. What energy must ...
Chapter 10
... Predicting Products of Synthesis Reactions For Synthesis Reactions For metals that only form one cation, determine the charge on the ion of each element (metallic and nonmetallic) and form a compound from the two ions. If one of the elements forms more than one cation or 2 nonmetals are combined ...
... Predicting Products of Synthesis Reactions For Synthesis Reactions For metals that only form one cation, determine the charge on the ion of each element (metallic and nonmetallic) and form a compound from the two ions. If one of the elements forms more than one cation or 2 nonmetals are combined ...
Syntheses of Soluble, -Stacking Tetracene Derivatives π
... 5d, respectively. We found that the introduction of both fluorine and a hexyloxy group offered a slight change on oxidation potentials, as compared to the E1/2 potential of tetracene. However, 5c and 5d exhibited relatively higher oxidation potentials due to the modest electron-donating ability of t ...
... 5d, respectively. We found that the introduction of both fluorine and a hexyloxy group offered a slight change on oxidation potentials, as compared to the E1/2 potential of tetracene. However, 5c and 5d exhibited relatively higher oxidation potentials due to the modest electron-donating ability of t ...
CHEMISTRY ANSWERS TO Textbook Questions
... 5. (a) The dimensions of the fabric change without altering its composition. (b) The salad is a mixture of ingredients. It does not alter the composition of any one ingredient. (c) None of the components in the lawnmower had their original composition changed. They were simply rearranged into a boat ...
... 5. (a) The dimensions of the fabric change without altering its composition. (b) The salad is a mixture of ingredients. It does not alter the composition of any one ingredient. (c) None of the components in the lawnmower had their original composition changed. They were simply rearranged into a boat ...
File
... Physical change: a change that doesn’t create a new kind of particle (chemical). They are often easy to reverse. Ex. Cutting, melting, boiling, dissolving etc. Chemical Change: a change that does create a new kind of particle (chemical). Indicators of chemical change 1. heat is produced or absor ...
... Physical change: a change that doesn’t create a new kind of particle (chemical). They are often easy to reverse. Ex. Cutting, melting, boiling, dissolving etc. Chemical Change: a change that does create a new kind of particle (chemical). Indicators of chemical change 1. heat is produced or absor ...
Elements and Minerals
... • please make sure you attend the class and talk with your TA about what is expected II. Today: From the “big picture” to the VERY small one! • from large-scale hazards to the scale of atoms • critical for understanding the building blocks of geology • we will not spend as much time on this subject ...
... • please make sure you attend the class and talk with your TA about what is expected II. Today: From the “big picture” to the VERY small one! • from large-scale hazards to the scale of atoms • critical for understanding the building blocks of geology • we will not spend as much time on this subject ...
Optical Properties
... “Define a metal” You will find that the answer is not as commonplace as the material itself! The answer is “Metals are materials with a positive thermal coefficient of resistivity”. What this means is that when the temperature of the metal is raised, its resistance increases. Other materials which c ...
... “Define a metal” You will find that the answer is not as commonplace as the material itself! The answer is “Metals are materials with a positive thermal coefficient of resistivity”. What this means is that when the temperature of the metal is raised, its resistance increases. Other materials which c ...
- Gondwana University, Gadchiroli
... A) Ionic Solids: Ionic structures, radius ratio effect & coordination number, Limitation of radius ratio rule, Lattice energy and Born-Haber cycle. Salvation energy and solubility of ionic solids, polarizing power and polarizability of ions, Fajan’s rules. [6L] ...
... A) Ionic Solids: Ionic structures, radius ratio effect & coordination number, Limitation of radius ratio rule, Lattice energy and Born-Haber cycle. Salvation energy and solubility of ionic solids, polarizing power and polarizability of ions, Fajan’s rules. [6L] ...
TM - Intro to Organi..
... Organic Chemistry • Carbon has the ability to form long chains. • Without this property, large biomolecules such as proteins, lipids, carbohydrates, and nucleic acids could not form. • Carbon easily forms bonds with other non-metal atoms. ...
... Organic Chemistry • Carbon has the ability to form long chains. • Without this property, large biomolecules such as proteins, lipids, carbohydrates, and nucleic acids could not form. • Carbon easily forms bonds with other non-metal atoms. ...
Phase composition of silver-doped hydroxyapatite depending on
... fields for a long time. It is well known that silver and silver-based compounds are antimicrobial to as many as 16 kinds of bacteria [1]. The combination of silver and hydroxyapatite (HAp/Ag) is used in the biomedical applications, due to their osteoconductivity and bioactivity [2]. In this study mo ...
... fields for a long time. It is well known that silver and silver-based compounds are antimicrobial to as many as 16 kinds of bacteria [1]. The combination of silver and hydroxyapatite (HAp/Ag) is used in the biomedical applications, due to their osteoconductivity and bioactivity [2]. In this study mo ...























