Review - Final Exam
... 26. Which has a larger second ionization energy, Mg or Na? Explain why. 27. What happens to the size of an atom when electrons are lost and gained? Explain why. 28. Write the formulas for two positive ions and two negative ions that have the same number of electrons as Ne and arrange them in order f ...
... 26. Which has a larger second ionization energy, Mg or Na? Explain why. 27. What happens to the size of an atom when electrons are lost and gained? Explain why. 28. Write the formulas for two positive ions and two negative ions that have the same number of electrons as Ne and arrange them in order f ...
Ch.1-Matter and Change
... Basic Building Blocks of Matter An atom is the smallest unit of an element that maintains the chemical identity of that element. An element is a pure substance that cannot be broken down into simpler, stable substances and is made of one type of atom. A compound is a substance that can be broken dow ...
... Basic Building Blocks of Matter An atom is the smallest unit of an element that maintains the chemical identity of that element. An element is a pure substance that cannot be broken down into simpler, stable substances and is made of one type of atom. A compound is a substance that can be broken dow ...
Exam Review – Part 1
... A substance that produces hydroxide ions, OH - (aq), when it dissolves in water. Bitter-tasting, slippery-feeling compounds; good conductors of electricity. E.g., Sodium hydroxide: NaOH (aq) NH4OH (aq) ...
... A substance that produces hydroxide ions, OH - (aq), when it dissolves in water. Bitter-tasting, slippery-feeling compounds; good conductors of electricity. E.g., Sodium hydroxide: NaOH (aq) NH4OH (aq) ...
Course Pack3 Phase Diagrams
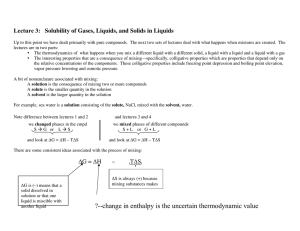
... ∆Hsoln is (+) for NaCl in H2O ∆Hsoln is (–) for Na2SO4 in H2O ∆Hsoln is (–) for O2 in H2O Consider the case that ∆Hmix is negative: since ∆Smix is positive then ∆Gsoln will have to be negative and the reaction happens. Now consider the case that ∆Hmix is positive: in this case the spontaneity of the ...
... ∆Hsoln is (+) for NaCl in H2O ∆Hsoln is (–) for Na2SO4 in H2O ∆Hsoln is (–) for O2 in H2O Consider the case that ∆Hmix is negative: since ∆Smix is positive then ∆Gsoln will have to be negative and the reaction happens. Now consider the case that ∆Hmix is positive: in this case the spontaneity of the ...
Properties of Minerals - Red Hook Central School District
... They combine in a specific structure called a: ...
... They combine in a specific structure called a: ...
Slide 1 - Effingham County Schools
... • Aristotle was wrong. However, his theory persisted for 2000 years. ...
... • Aristotle was wrong. However, his theory persisted for 2000 years. ...
Elements Combine to Form Compounds
... a change in matter in which NEW substances are produced with NEW properties. Clues that May Indicate a Chemical Change ...
... a change in matter in which NEW substances are produced with NEW properties. Clues that May Indicate a Chemical Change ...
p131
... requires the participation of researchers in both industry and academia. The preferred route of administration of most drugs is in oral dosage forms with the active ingredient as a crystalline solid. The production of crystalline active pharmaceutical ingredients (API) by crystallization is therefor ...
... requires the participation of researchers in both industry and academia. The preferred route of administration of most drugs is in oral dosage forms with the active ingredient as a crystalline solid. The production of crystalline active pharmaceutical ingredients (API) by crystallization is therefor ...
Naming Compounds
... a change in matter in which NEW substances are produced with NEW properties. Clues that May Indicate a Chemical Change ...
... a change in matter in which NEW substances are produced with NEW properties. Clues that May Indicate a Chemical Change ...
2.5 THE NAMES AND FORMULAS OF COMPOUNDS
... explain many of the properties of ionic compounds, but they aren’t sufficient to explain the physical state of molecular compounds. If covalent bonds were the only forces at work, molecular compounds would all be gases, as there would be no attraction between the molecules strong enough to order the ...
... explain many of the properties of ionic compounds, but they aren’t sufficient to explain the physical state of molecular compounds. If covalent bonds were the only forces at work, molecular compounds would all be gases, as there would be no attraction between the molecules strong enough to order the ...
110 mid-term 2011Solutions
... Incoming X-ray radiation interact with inner-shell electrons, causing an excitation to a higher level. These excitations relax back down to a lower, possibly not ground state, energy level – the energy difference is emitted as an emission particle with X-ray energy lower than the incident but specif ...
... Incoming X-ray radiation interact with inner-shell electrons, causing an excitation to a higher level. These excitations relax back down to a lower, possibly not ground state, energy level – the energy difference is emitted as an emission particle with X-ray energy lower than the incident but specif ...
Lecture Notes
... which a substance changes from the solid state to the liquid state. For pure water themelting point is 32oF or 0oC. The Freezing point of a substance is the same as the melting point, since the process of freezing is the opposite of melting. The boiling point is defined as that temperature at which ...
... which a substance changes from the solid state to the liquid state. For pure water themelting point is 32oF or 0oC. The Freezing point of a substance is the same as the melting point, since the process of freezing is the opposite of melting. The boiling point is defined as that temperature at which ...
The Atom Power point - Effingham County Schools
... • Aristotle was wrong. However, his theory persisted for 2000 years. ...
... • Aristotle was wrong. However, his theory persisted for 2000 years. ...
Chapter 1 Matter and Change
... States of matter 1) Solid- matter that can not flow (definite shape) and has definite volume. 2) Liquid- definite volume but takes the shape of its container (flows). 3) Gas- a substance without definite volume or shape and can flow. – Vapor- a substance that is currently a gas, but normally is a l ...
... States of matter 1) Solid- matter that can not flow (definite shape) and has definite volume. 2) Liquid- definite volume but takes the shape of its container (flows). 3) Gas- a substance without definite volume or shape and can flow. – Vapor- a substance that is currently a gas, but normally is a l ...
CHM 130 Final Exam Review Chapter 1 Scientific method Theory
... Electron dot formulas Shapes of molecules (table given) Polar vs nonpolar covalent bonds Metallic bonds Polar vs nonpolar molecules Chapter 7 Ion charges (oxidation states) Writing ionic formulas ...
... Electron dot formulas Shapes of molecules (table given) Polar vs nonpolar covalent bonds Metallic bonds Polar vs nonpolar molecules Chapter 7 Ion charges (oxidation states) Writing ionic formulas ...
CHM 130 Final Exam Review
... Electron dot formulas Shapes of molecules (table given) Polar vs nonpolar covalent bonds Metallic bonds Polar vs nonpolar molecules ...
... Electron dot formulas Shapes of molecules (table given) Polar vs nonpolar covalent bonds Metallic bonds Polar vs nonpolar molecules ...
Methods of solubility improvements
... liquid and boil them away into a gas. Only when the liquid has been completely vaporized will the temperature of the system again begin to rise. If we measure the amount of energy (in calories) that was added from the onset of boiling to the point when all the liquid has boiled away, we will have a ...
... liquid and boil them away into a gas. Only when the liquid has been completely vaporized will the temperature of the system again begin to rise. If we measure the amount of energy (in calories) that was added from the onset of boiling to the point when all the liquid has boiled away, we will have a ...
Ionic Bonding
... 7. Water is known for its many anomalous properties. Use your knowledge of intermolecular forces and intramolecular bonding to explain theoretically why lakes freeze from top to bottom. 8. Using Table 3 (page 85), predict whether each of the following moleculeswould be polar or nonpolar. (a) CH3OH(l ...
... 7. Water is known for its many anomalous properties. Use your knowledge of intermolecular forces and intramolecular bonding to explain theoretically why lakes freeze from top to bottom. 8. Using Table 3 (page 85), predict whether each of the following moleculeswould be polar or nonpolar. (a) CH3OH(l ...
Matter Test Review Sheet
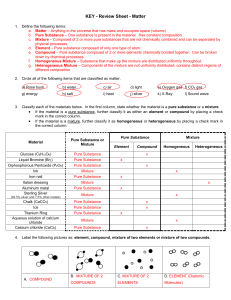
... b. Pure Substance – One substance is present in the material. Has constant composition. c. Mixture – Composed of 2 or more pure substances that are not chemically combined and can be separated by physical processes. d. Element – Pure substance composed of only one type of atom. e. Compound – Pure su ...
... b. Pure Substance – One substance is present in the material. Has constant composition. c. Mixture – Composed of 2 or more pure substances that are not chemically combined and can be separated by physical processes. d. Element – Pure substance composed of only one type of atom. e. Compound – Pure su ...
Chapter 1 Chemistry: The Study of Matter
... Nuclear Energy – Energy from changing the nucleus of atoms All types of energy can be converted into others. If you trace the source far enough back, you will end up at nuclear energy. ...
... Nuclear Energy – Energy from changing the nucleus of atoms All types of energy can be converted into others. If you trace the source far enough back, you will end up at nuclear energy. ...
Unit 3 Practice Test
... Numerical Response 3. When the molecular substances that have hydrogen bondings present in the solid or liquid states are listed by number, in numerical order, the sequence of numbers is ____, ____, ____, ____. ...
... Numerical Response 3. When the molecular substances that have hydrogen bondings present in the solid or liquid states are listed by number, in numerical order, the sequence of numbers is ____, ____, ____, ____. ...
Chapter 2
... • Solids have rigid structure. • Liquids take the shape of the container. • Energy is the key! ...
... • Solids have rigid structure. • Liquids take the shape of the container. • Energy is the key! ...
Earth’s Materials - Lower Hudson Regional Information Center
... The color of a mineral can be useful, HOWEVER, it can vary due to slight chemical differences The streak is the color of freshly crushed mineral powder and is usually constant. ...
... The color of a mineral can be useful, HOWEVER, it can vary due to slight chemical differences The streak is the color of freshly crushed mineral powder and is usually constant. ...
Here - Liquid Crystals and Photonics Group
... Liquid crystals are widely used in display devices but their electro-optical properties can also be used in many other applications. In smart phones the autofocus is based on a small lens that can move with respect to the camera, and it would be more reliable to use an electronic component without m ...
... Liquid crystals are widely used in display devices but their electro-optical properties can also be used in many other applications. In smart phones the autofocus is based on a small lens that can move with respect to the camera, and it would be more reliable to use an electronic component without m ...