2007 National Qualifying Exam – Chemistry
... compound containing one bromine atom will give rise to two molecular peaks depending on which isotope it contains. One peak will result from molecular ions containing 79Br, and the other will result from molecular ions containing 81Br. The two peaks differ by two m/z units. The relative intensities ...
... compound containing one bromine atom will give rise to two molecular peaks depending on which isotope it contains. One peak will result from molecular ions containing 79Br, and the other will result from molecular ions containing 81Br. The two peaks differ by two m/z units. The relative intensities ...
Electronic Structure of Cobalt Nanocrystals Suspended in Liquid
... field split d-levels.21,22 The relative intensity of the peak shows a notable resonance effect with incident energy. Another set of Raman peaks occurs at -6.7 eV, which corresponds to the ligand O 2p - Co 3d charge-transfer excitations. To understand these spectroscopic observations, the singleimpur ...
... field split d-levels.21,22 The relative intensity of the peak shows a notable resonance effect with incident energy. Another set of Raman peaks occurs at -6.7 eV, which corresponds to the ligand O 2p - Co 3d charge-transfer excitations. To understand these spectroscopic observations, the singleimpur ...
Excited States of Pt(PF3)4 and Their Role in Focused Electron Beam
... changes in EPauli and Eelst, their sum being less destabilizing than in the previous two cases. Finally, in the case of the elimination of the fourth ligand, EPauli, Eelst, and Eorb have significantly higher values because of the shortest Pt−P distance, 0.2 Å shorter than in Pt(PF3)4. Change of BDE i ...
... changes in EPauli and Eelst, their sum being less destabilizing than in the previous two cases. Finally, in the case of the elimination of the fourth ligand, EPauli, Eelst, and Eorb have significantly higher values because of the shortest Pt−P distance, 0.2 Å shorter than in Pt(PF3)4. Change of BDE i ...
Coordination Nature of 4-Mercaptoaniline to Sn(II) Ion: Formation of
... impure product containing compounds 1 and 2. Route 2: 0.0509 g SnCl4·5H2O (0.1 mmol) was combined with 0.0736 g (0.6 mmol) ethanolic 4-MA in a vial. A yellow solution forms and, upon further disturbance, small yellow crystals precipitate from solution. This mixture was dried under vacuum then washed ...
... impure product containing compounds 1 and 2. Route 2: 0.0509 g SnCl4·5H2O (0.1 mmol) was combined with 0.0736 g (0.6 mmol) ethanolic 4-MA in a vial. A yellow solution forms and, upon further disturbance, small yellow crystals precipitate from solution. This mixture was dried under vacuum then washed ...
Module 3 Transition elements - Pearson Schools and FE Colleges
... configuration of 1s22s22p63s23p64s23d6. • To form the 3+ ion an iron atom has to lose three electrons. • Fe3+ will have an electron configuration of 1s22s22p63s23p63d5. • Fe has lost two electrons from the 4s sub-shell and one electron from the d sub-shell. A copper atom has 29 electrons and its ...
... configuration of 1s22s22p63s23p64s23d6. • To form the 3+ ion an iron atom has to lose three electrons. • Fe3+ will have an electron configuration of 1s22s22p63s23p63d5. • Fe has lost two electrons from the 4s sub-shell and one electron from the d sub-shell. A copper atom has 29 electrons and its ...
Nickel macrocycles with complex hydrides—new avenues for
... the abundant possibilities for designing transition metal borohydride complexes. Regarding the desired catalytic role, it doesn’t seem unreasonable (though recognising this as speculation in the absence of a definite mechanism) that when either discharging or recharging the hydridic hydrogen store t ...
... the abundant possibilities for designing transition metal borohydride complexes. Regarding the desired catalytic role, it doesn’t seem unreasonable (though recognising this as speculation in the absence of a definite mechanism) that when either discharging or recharging the hydridic hydrogen store t ...
Some Reactions Involving [W(N-2,6-Me C H )(OCMe
... with a (W-W)10+ core which contains a formal d1-d1 M-M single bond,9a,c in agreement with the observed diamagnetism of the compound. Lengthening of the W-N bonds by ∼0.2 Å and the decrease in W-N-C bond angles (to ∼135°) relative to those of the parent compound (WdN ) 1.753(3) Å and WdN-C ) 172.1(3) ...
... with a (W-W)10+ core which contains a formal d1-d1 M-M single bond,9a,c in agreement with the observed diamagnetism of the compound. Lengthening of the W-N bonds by ∼0.2 Å and the decrease in W-N-C bond angles (to ∼135°) relative to those of the parent compound (WdN ) 1.753(3) Å and WdN-C ) 172.1(3) ...
Sn19.3Cu4.7As22I8: a New Clathrate-I
... Sn19.3(4)Cu4.7(4)As22I8, which is in good agreement with the chemical analysis data. Found: 35.4(5):10.3(3):40.6(3):13.7(2). Calculated for Sn19.3(4)Cu4.7(4)As22I8: 35.7(7):8.7(7):40.7: 14.8. All attempts to describe the electron density near the 24k site as two instead of three neighboring metal po ...
... Sn19.3(4)Cu4.7(4)As22I8, which is in good agreement with the chemical analysis data. Found: 35.4(5):10.3(3):40.6(3):13.7(2). Calculated for Sn19.3(4)Cu4.7(4)As22I8: 35.7(7):8.7(7):40.7: 14.8. All attempts to describe the electron density near the 24k site as two instead of three neighboring metal po ...
Radical reactions with metal complexes in aqueous solutions
... radicals will be converted into charged entities due to the electron transfer and thus intrinsically have distinctly higher solvation energy ...
... radicals will be converted into charged entities due to the electron transfer and thus intrinsically have distinctly higher solvation energy ...
Document
... • Dz2 and dx2-y2 orbitals have lobes that point directly at the ligands. • Dxy, dyz,dxz point their lobes between charges. • Electrons fill the d orbitals farthest from the ligands to minimize repulsion. • Dxy, dyz,dxz ( t2g set) are at lower energy in octahedral complex first. • Dz2 and dx2-y2 (eg ...
... • Dz2 and dx2-y2 orbitals have lobes that point directly at the ligands. • Dxy, dyz,dxz point their lobes between charges. • Electrons fill the d orbitals farthest from the ligands to minimize repulsion. • Dxy, dyz,dxz ( t2g set) are at lower energy in octahedral complex first. • Dz2 and dx2-y2 (eg ...
Arylboronates and Diarylborinates
... and form the rhodium boronate complexes 3a-e. The parasubstituted aryl derivatives 3a-d were stable enough to be obtained as crystalline solids in 45-76% isolated yields and were fully characterized. The o-anisyl derivative 3e was unstable at room temperature, but it formed in quantitative yield at ...
... and form the rhodium boronate complexes 3a-e. The parasubstituted aryl derivatives 3a-d were stable enough to be obtained as crystalline solids in 45-76% isolated yields and were fully characterized. The o-anisyl derivative 3e was unstable at room temperature, but it formed in quantitative yield at ...
Mercury Complexes Derived From Some Acetone
... (Table 1). The ferromagnetism and antiferromagnetism effect indicate in some extent, there are some intermolecular and/or intramolecular magnetic interaction.23 3.3. Infrared Spectra The significant ir data of the ligands as well as those of their mercury(I) and mercury(II) complexes are listed in T ...
... (Table 1). The ferromagnetism and antiferromagnetism effect indicate in some extent, there are some intermolecular and/or intramolecular magnetic interaction.23 3.3. Infrared Spectra The significant ir data of the ligands as well as those of their mercury(I) and mercury(II) complexes are listed in T ...
Metal Interactions in Chromatography
... Tetracycline is a member of a group of antibiotic drugs commonly used in human and veterinary medicine. The molecule has three potential chelating sites for iron aligned at one site. The drugs are known to bind metal ions as dietary calcium and iron can render them ineffective. ...
... Tetracycline is a member of a group of antibiotic drugs commonly used in human and veterinary medicine. The molecule has three potential chelating sites for iron aligned at one site. The drugs are known to bind metal ions as dietary calcium and iron can render them ineffective. ...
Shielding vs. Deshielding
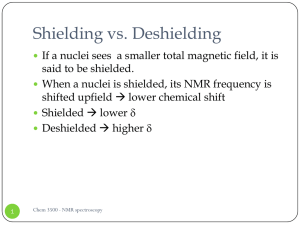
... When a nuclei is shielded, its NMR frequency is shifted upfield lower chemical shift ...
... When a nuclei is shielded, its NMR frequency is shifted upfield lower chemical shift ...
MO tutorials
... (LUMO). This has π symmetry i.e. has one nodal plane passing through the internuclear axis. To show the symmetry of the HOMO and LUMO you need to draw them and a really good answer will show the completed molecular orbital energy level diagram for CO, to prove these are the fully occupied and empty ...
... (LUMO). This has π symmetry i.e. has one nodal plane passing through the internuclear axis. To show the symmetry of the HOMO and LUMO you need to draw them and a really good answer will show the completed molecular orbital energy level diagram for CO, to prove these are the fully occupied and empty ...
Spin crossover

Spin Crossover (SCO), sometimes referred to as spin transition or spin equilibrium behavior, is a phenomenon that occurs in some metal complexes wherein the spin state of the complex changes due to external stimuli such as a variation of temperature, pressure, light irradiation or an influence of a magnetic field.With regard to a ligand field and ligand field theory, the change in spin state is a transition from a low spin (LS) ground state electron configuration to a high spin (HS) ground state electron configuration of the metal’s d atomic orbitals (AOs), or vice versa. The magnitude of the ligand field splitting along with the pairing energy of the complex determines whether it will have a LS or HS electron configuration. A LS state occurs because the ligand field splitting (Δ) is greater than the pairing energy of the complex (which is an unfavorable process).Figure 1 is a simplified illustration of the metal’s d orbital splitting in the presence of an octahedral ligand field. A large splitting between the t2g and eg AOs requires a substantial amount of energy for the electrons to overcome the energy gap (Δ) to comply with Hund’s Rule. Therefore, electrons will fill the lower energy t2g orbitals completely before populating the higher energy eg orbitals. Conversely, a HS state occurs with weaker ligand fields and smaller orbital splitting. In this case the energy required to populate the higher levels is substantially less than the pairing energy and the electrons fill the orbitals according to Hund’s Rule by populating the higher energy orbitals before pairing with electrons in the lower lying orbitals. An example of a metal ion that can exist in either a LS or HS state is Fe3+ in an octahedral ligand field. Depending on the ligands that are coordinated to this complex the Fe3+ can attain a LS or a HS state, as in Figure 1.Spin crossover refers to the transitions between high to low, or low to high, spin states. This phenomenon is commonly observed with some first row transition metal complexes with a d4 through d7 electron configuration in an octahedral ligand geometry. Spin transition curves are a common representation of SCO phenomenon with the most commonly observed types depicted in Figure 2 in which γHS (the high-spin molar fraction) is plotted vs. T. The figure shows a gradual spin transition (left), an abrupt transition with hysteresis (middle) and a two-step transition (right). For a transition to be considered gradual, it typically takes place over a large temperature range, even up to several hundred K, whereas for a transition to be considered abrupt, it should take place within 10 K or less.These curves indicate that a spin transition has occurred in a metal complex as temperature changed. The gradual transition curve is an indication that not all metal centers within the complex are undergoing the transition at the same temperature. The abrupt spin change with hysteresis indicates a strong cooperativity, or “communication”, between neighboring metal complexes. In the latter case, the material is bistable and can exist in the two different spin states with a different range of external stimuli (temperature in this case) for the two phenomena, namely LS → HS and HS → LS. The two-step transition is relatively rare but is observed, for example, with dinuclear SCO complexes for which the spin transition in one metal center renders the transition in the second metal center less favorable.There are several types of spin crossover that can occur in a complex; some of them are light induced excited state spin trapping (LIESST), ligand-driven light induced spin change (LD-LISC), and charge transfer induced spin transition (CTIST).