Bonding, aromaticity and reactivity patterns in some all
... (Δω) values. Theoretical background of the present work is presented in Section 2 and section 3 provides the numerical details. Section 4 presents the results and discussion and finally, section 5 contains ...
... (Δω) values. Theoretical background of the present work is presented in Section 2 and section 3 provides the numerical details. Section 4 presents the results and discussion and finally, section 5 contains ...
Orbital hybridisation From Wikipedia, the free encyclopedia Jump to
... hybridisation theory is not as practical as molecular orbital theory. Problems with hybridisation are especially notable when the d orbitals are involved in bonding, as in coordination chemistry and organometallic chemistry. Although hybridisation schemes in transition metal chemistry can be used, t ...
... hybridisation theory is not as practical as molecular orbital theory. Problems with hybridisation are especially notable when the d orbitals are involved in bonding, as in coordination chemistry and organometallic chemistry. Although hybridisation schemes in transition metal chemistry can be used, t ...
MANE6960 Homework05 Wyatt.pdf
... Thermal and prompt NO are formed by the combustion of clean fuels (which do not contain nitrogen compounds) with air (which contains atmospheric N2). NO is formed mainly by the Zel’dovich mechanism [1]. The Zel’dovich mechanism requires breaking of the strong triple bond in the N2 moelcule, hence th ...
... Thermal and prompt NO are formed by the combustion of clean fuels (which do not contain nitrogen compounds) with air (which contains atmospheric N2). NO is formed mainly by the Zel’dovich mechanism [1]. The Zel’dovich mechanism requires breaking of the strong triple bond in the N2 moelcule, hence th ...
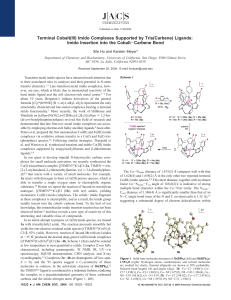
Terminal Cobalt(III) Imido Complexes Supported by Tris(Carbene
... characterized, including paramagnetic 1H NMR, IR, UV-vis spectroscopy, SQUID measurements, CHN analysis, and X-ray crystallography.12 Complexes 3a-4b are diamagnetic (d6 low-spin, S ) 0), and the 1H spectra suggest a C3-symmetry of these molecules in solution. In the solid-state structure of 3b(BPh4 ...
... characterized, including paramagnetic 1H NMR, IR, UV-vis spectroscopy, SQUID measurements, CHN analysis, and X-ray crystallography.12 Complexes 3a-4b are diamagnetic (d6 low-spin, S ) 0), and the 1H spectra suggest a C3-symmetry of these molecules in solution. In the solid-state structure of 3b(BPh4 ...
Electron Paramagnetic Resonance Theory E.C. Duin
... motions averages the anisotropy of the g-factor resulting in the detection of a narrow isotropic EPR signal with one apparent g-value, giso. In this case the splitting patterns will all have their origin in this central isotropic signal. • With large molecules like proteins that show slow tumbling, ...
... motions averages the anisotropy of the g-factor resulting in the detection of a narrow isotropic EPR signal with one apparent g-value, giso. In this case the splitting patterns will all have their origin in this central isotropic signal. • With large molecules like proteins that show slow tumbling, ...
Photo chapter opener 20 INSERT CAPTION
... approach is to use them if the name of the ligand is three or more syllables long! Copyright © Houghton Mifflin Company. All rights reserved. ...
... approach is to use them if the name of the ligand is three or more syllables long! Copyright © Houghton Mifflin Company. All rights reserved. ...
Bioinspired Infrared Detection Using Thermoresponsive
... 120-nm NPs; (b) 170-nm NPs; (c) 300-nm NPs. The 1H NMR spectra of the hydrogel NPs were recorded on a Bruker BioSpin GmbH 600 MHz NMR spectrometer using D2O as the solvent. As shown in Figure S2, the NMR spectra for all the NP samples are similar, which is also consistent with the reported spectra f ...
... 120-nm NPs; (b) 170-nm NPs; (c) 300-nm NPs. The 1H NMR spectra of the hydrogel NPs were recorded on a Bruker BioSpin GmbH 600 MHz NMR spectrometer using D2O as the solvent. As shown in Figure S2, the NMR spectra for all the NP samples are similar, which is also consistent with the reported spectra f ...
Chem 4B First Midterm Review Sheet
... Ion exchange resins are insoluble materials that contain cations or anions that can be exchanged. The resins typically consist of a framework held together by strong chemical bonds. Positively or negatively charged functional groups are attached to this framework and each of these groups carries an ...
... Ion exchange resins are insoluble materials that contain cations or anions that can be exchanged. The resins typically consist of a framework held together by strong chemical bonds. Positively or negatively charged functional groups are attached to this framework and each of these groups carries an ...
Dinesh-ohiostate06
... The doublet appears but is redshifted further and the relative intensities are changed. ...
... The doublet appears but is redshifted further and the relative intensities are changed. ...
set of 39 exercises
... Is this further reduction in bond angle due to increased size of lone pairs on the heavy elements or are other factors involved? Do electrostatics (Coulomb’s law) or changes in orbital hybridization play a role? a. Optimize the geometries of the eight hydrides shown above using the HF/3-21G model an ...
... Is this further reduction in bond angle due to increased size of lone pairs on the heavy elements or are other factors involved? Do electrostatics (Coulomb’s law) or changes in orbital hybridization play a role? a. Optimize the geometries of the eight hydrides shown above using the HF/3-21G model an ...
Chemistry 213 - Winona State University
... ethylenediamine ligands would force a symmetrical structure, as in the nickel complex, since the length of the en skeleton is fixed. It remains to be seen whether the symmetry requirement will overcome the electronic factors. The purpose of this experiment is to determine which of the copper complex ...
... ethylenediamine ligands would force a symmetrical structure, as in the nickel complex, since the length of the en skeleton is fixed. It remains to be seen whether the symmetry requirement will overcome the electronic factors. The purpose of this experiment is to determine which of the copper complex ...
Polynuclear Complexes Containing Ditopic Bispyrazolylmethane
... developed and this involves the synthesis of molecular building blocks that are themselves metal complexes. In this way, a metalloligand is synthesized initially and this compound is subsequently added to another metal ion, which acts as a node in the framework and, consequently, two kinds of metal ...
... developed and this involves the synthesis of molecular building blocks that are themselves metal complexes. In this way, a metalloligand is synthesized initially and this compound is subsequently added to another metal ion, which acts as a node in the framework and, consequently, two kinds of metal ...
AND BINUCLEAR COMPLEXES CONTAINING OXIME LIGANDS
... Spectrum-100 Perkin Elmer spectrometer in the range of 400-4000 cm-1. X-ray data were collected at room temperature on an Oxford Diffraction Xcalibur diffractometer equipped with CCD area detector and a graphite monochromator utilizing MoKα radiation. Final unit cell dimensions were obtained and ref ...
... Spectrum-100 Perkin Elmer spectrometer in the range of 400-4000 cm-1. X-ray data were collected at room temperature on an Oxford Diffraction Xcalibur diffractometer equipped with CCD area detector and a graphite monochromator utilizing MoKα radiation. Final unit cell dimensions were obtained and ref ...
full paper - Wayne State Chemistry Department
... (Scheme 1). Compounds 1⫺3 are air-sensitive in solution and in the solid state, but are thermally stable indefinitely at ambient temperature under argon. The structures of 1⫺3 were assigned by 1H NMR spectroscopy, IR spectroscopy, microanalysis, and by X-ray structure determinations (vide infra). Co ...
... (Scheme 1). Compounds 1⫺3 are air-sensitive in solution and in the solid state, but are thermally stable indefinitely at ambient temperature under argon. The structures of 1⫺3 were assigned by 1H NMR spectroscopy, IR spectroscopy, microanalysis, and by X-ray structure determinations (vide infra). Co ...
carbwagn.a01.pub2 1..4
... Hydroformylation and Hydrogenation. Hydroformylation (or the OXO process) involves the addition of hydrogen and carbon monoxide to an unsaturated organic substrate and it is effectively catalysed by a number of metal carbonyls, in particular Co2(CO)8 and its phosphine derivatives and various rhodium ...
... Hydroformylation and Hydrogenation. Hydroformylation (or the OXO process) involves the addition of hydrogen and carbon monoxide to an unsaturated organic substrate and it is effectively catalysed by a number of metal carbonyls, in particular Co2(CO)8 and its phosphine derivatives and various rhodium ...
Lecture 26
... coordination number) The stabilization of a metal complex by a ligand with more than one donor atom is known as the chelate effect. ...
... coordination number) The stabilization of a metal complex by a ligand with more than one donor atom is known as the chelate effect. ...
Oxidation Chemistry of Metal(II) Salen-Type Complexes
... especially 5-membered Ni and Pt chelate complexes, only from the X-ray crystal structure analysis may be difficult, since we can detect mainly the contraction of the coordination sphere, which is predicted to be also observed in high valent metal salen-type complexes. On the other hand, the electron ...
... especially 5-membered Ni and Pt chelate complexes, only from the X-ray crystal structure analysis may be difficult, since we can detect mainly the contraction of the coordination sphere, which is predicted to be also observed in high valent metal salen-type complexes. On the other hand, the electron ...
D AND F BLOCK ELEMENT
... (ii) Transition elements have high effective nuclear charge and a large number of valence electrons. Therefore, they form very strong metallic bonds. As a result, the enthalpy of atomization of transition metals is high. (iii) Most of the complexes of transition metals are coloured. This is because ...
... (ii) Transition elements have high effective nuclear charge and a large number of valence electrons. Therefore, they form very strong metallic bonds. As a result, the enthalpy of atomization of transition metals is high. (iii) Most of the complexes of transition metals are coloured. This is because ...
Spin crossover

Spin Crossover (SCO), sometimes referred to as spin transition or spin equilibrium behavior, is a phenomenon that occurs in some metal complexes wherein the spin state of the complex changes due to external stimuli such as a variation of temperature, pressure, light irradiation or an influence of a magnetic field.With regard to a ligand field and ligand field theory, the change in spin state is a transition from a low spin (LS) ground state electron configuration to a high spin (HS) ground state electron configuration of the metal’s d atomic orbitals (AOs), or vice versa. The magnitude of the ligand field splitting along with the pairing energy of the complex determines whether it will have a LS or HS electron configuration. A LS state occurs because the ligand field splitting (Δ) is greater than the pairing energy of the complex (which is an unfavorable process).Figure 1 is a simplified illustration of the metal’s d orbital splitting in the presence of an octahedral ligand field. A large splitting between the t2g and eg AOs requires a substantial amount of energy for the electrons to overcome the energy gap (Δ) to comply with Hund’s Rule. Therefore, electrons will fill the lower energy t2g orbitals completely before populating the higher energy eg orbitals. Conversely, a HS state occurs with weaker ligand fields and smaller orbital splitting. In this case the energy required to populate the higher levels is substantially less than the pairing energy and the electrons fill the orbitals according to Hund’s Rule by populating the higher energy orbitals before pairing with electrons in the lower lying orbitals. An example of a metal ion that can exist in either a LS or HS state is Fe3+ in an octahedral ligand field. Depending on the ligands that are coordinated to this complex the Fe3+ can attain a LS or a HS state, as in Figure 1.Spin crossover refers to the transitions between high to low, or low to high, spin states. This phenomenon is commonly observed with some first row transition metal complexes with a d4 through d7 electron configuration in an octahedral ligand geometry. Spin transition curves are a common representation of SCO phenomenon with the most commonly observed types depicted in Figure 2 in which γHS (the high-spin molar fraction) is plotted vs. T. The figure shows a gradual spin transition (left), an abrupt transition with hysteresis (middle) and a two-step transition (right). For a transition to be considered gradual, it typically takes place over a large temperature range, even up to several hundred K, whereas for a transition to be considered abrupt, it should take place within 10 K or less.These curves indicate that a spin transition has occurred in a metal complex as temperature changed. The gradual transition curve is an indication that not all metal centers within the complex are undergoing the transition at the same temperature. The abrupt spin change with hysteresis indicates a strong cooperativity, or “communication”, between neighboring metal complexes. In the latter case, the material is bistable and can exist in the two different spin states with a different range of external stimuli (temperature in this case) for the two phenomena, namely LS → HS and HS → LS. The two-step transition is relatively rare but is observed, for example, with dinuclear SCO complexes for which the spin transition in one metal center renders the transition in the second metal center less favorable.There are several types of spin crossover that can occur in a complex; some of them are light induced excited state spin trapping (LIESST), ligand-driven light induced spin change (LD-LISC), and charge transfer induced spin transition (CTIST).