Carboxylates/esters vs ketones/aldehydes
... The mechanism of the NaBH4 reduction in a protic solvent such as ethanol, methanol, and water is known to be quite complex since NaBH4 reacts with the solvent, e.g., NaBH4 + C2H5OH → NaBH3(OC2H5) + H2 Because of this, usually at least one mol. equivalents of NaBH4 are used for the reaction in a prot ...
... The mechanism of the NaBH4 reduction in a protic solvent such as ethanol, methanol, and water is known to be quite complex since NaBH4 reacts with the solvent, e.g., NaBH4 + C2H5OH → NaBH3(OC2H5) + H2 Because of this, usually at least one mol. equivalents of NaBH4 are used for the reaction in a prot ...
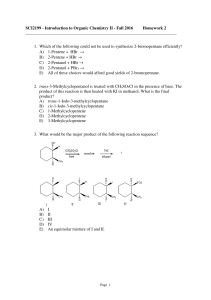
SCI2199 - Introduction to Organic Chemistry II
... 7. Which of the following could be used to synthesize 2-bromobutane? A) CH3CH2CH=CH2 + Br2(aq) → B) CH3CH2CHOHCH3 + HBr → C) CH3CH2C≡CH + HBr → D) CH3CH2C≡CH + Br2 → E) More than one of these choices. 8. Which of the alcohols listed below would you expect to react most rapidly with ...
... 7. Which of the following could be used to synthesize 2-bromobutane? A) CH3CH2CH=CH2 + Br2(aq) → B) CH3CH2CHOHCH3 + HBr → C) CH3CH2C≡CH + HBr → D) CH3CH2C≡CH + Br2 → E) More than one of these choices. 8. Which of the alcohols listed below would you expect to react most rapidly with ...
14875-46074-1
... TiCl4, SnCl4, AlCl3, Sn (OTf)2 and ZnBr2[1,7]. Montmorillonite K10 clay catalyst [8] and silica gel (under low pressure) [9] or thermolytic conditions at high temperature (150°C) [10] have also shown to work. Cleavage of the Boc group can also be achieved in some cases under basic conditions, where ...
... TiCl4, SnCl4, AlCl3, Sn (OTf)2 and ZnBr2[1,7]. Montmorillonite K10 clay catalyst [8] and silica gel (under low pressure) [9] or thermolytic conditions at high temperature (150°C) [10] have also shown to work. Cleavage of the Boc group can also be achieved in some cases under basic conditions, where ...
Chapter 23 Functional Groups
... Few halocarbons found in nature –but, readily prepared and used –halothane and also the ...
... Few halocarbons found in nature –but, readily prepared and used –halothane and also the ...
C07B - Cooperative Patent Classification
... GENERAL METHODS OF ORGANIC CHEMISTRY; APPARATUS THEREFOR (preparation of carboxylic acid esters by telomerisation C07C 67/47; telomerisation C08F) NOTES 1. In this subclass, the functional group which is present already in some residue being introduced and is not substantially involved in a chemical ...
... GENERAL METHODS OF ORGANIC CHEMISTRY; APPARATUS THEREFOR (preparation of carboxylic acid esters by telomerisation C07C 67/47; telomerisation C08F) NOTES 1. In this subclass, the functional group which is present already in some residue being introduced and is not substantially involved in a chemical ...
Organic #2
... Depending on reaction conditions, oxidation of butan-1-ol can give two different organic products. Name the functional group present in each of these products. Explain how you could distinguish between these two products. Name the reagents you would use and say what you would observe. What reagent w ...
... Depending on reaction conditions, oxidation of butan-1-ol can give two different organic products. Name the functional group present in each of these products. Explain how you could distinguish between these two products. Name the reagents you would use and say what you would observe. What reagent w ...
Development of Novel Catalytic Asymmetric Reactions using
... β-ketoesters.13 In this article, results in the case of N-Boc imine are disucussed in Table 1. Due to the imine activation by protic acid, the reaction is dramatically accelerated compared to the case of Michael addition. In many cases, the reactions were completed within several hours. β-Aminocarbo ...
... β-ketoesters.13 In this article, results in the case of N-Boc imine are disucussed in Table 1. Due to the imine activation by protic acid, the reaction is dramatically accelerated compared to the case of Michael addition. In many cases, the reactions were completed within several hours. β-Aminocarbo ...
Efficient and catalyst-free condensation of acid chlorides and
... At last, the condensation between an acid chloride (4-BrC6H4COCl) and an alcohol (p-cresol) was evaluated when both reagents were dissolved in CH3CN. The reactions were performed at a temperature of 140°C and a residence time of 400 sec. Initial experiments revealed that 2 equivalents of p-cresol ar ...
... At last, the condensation between an acid chloride (4-BrC6H4COCl) and an alcohol (p-cresol) was evaluated when both reagents were dissolved in CH3CN. The reactions were performed at a temperature of 140°C and a residence time of 400 sec. Initial experiments revealed that 2 equivalents of p-cresol ar ...
M_ScOrganic_Chemistr..
... Organic synthesis: Reterosynthsis, synthons and synthetic equivalents, functional group interconversion, Synthesis of amines, regiospecific, chemospecific and stereospecific reactions, umpolung methods. principles and applications of protective groups in protection of hydroxyl, amino, carbonyl and c ...
... Organic synthesis: Reterosynthsis, synthons and synthetic equivalents, functional group interconversion, Synthesis of amines, regiospecific, chemospecific and stereospecific reactions, umpolung methods. principles and applications of protective groups in protection of hydroxyl, amino, carbonyl and c ...
ADVANCED SYNTHESIS Stereochemistry
... • Allylic alcohols undergo diastereoselective epoxidation • If we use a free hydroxyl group then the peracid hydrogen bonds to the hydroxyl group • Results in a pseudo-intramolecular reaction • If alcohol is protected then epoxidation occurs from least hindered face OH ...
... • Allylic alcohols undergo diastereoselective epoxidation • If we use a free hydroxyl group then the peracid hydrogen bonds to the hydroxyl group • Results in a pseudo-intramolecular reaction • If alcohol is protected then epoxidation occurs from least hindered face OH ...
czesc_2.chp:Corel VENTURA
... mixture containing DBM has an irregular shape and soft gel structure and therefore it is not further analyzed. The other samples are rigid spheres with diameter of 0.1—0.25 mm. The nitrogen concentrations in all MIP samples are lower than nominal values in both nitrogen containing monomers, which pr ...
... mixture containing DBM has an irregular shape and soft gel structure and therefore it is not further analyzed. The other samples are rigid spheres with diameter of 0.1—0.25 mm. The nitrogen concentrations in all MIP samples are lower than nominal values in both nitrogen containing monomers, which pr ...
F324 : Rings, Polymers and Analysis
... Candidates should be able to: (a) describe the oxidation of alcohols (see also unit F322: 2.2.1.f) using Cr2O72-/H+ (ie K2Cr2O7/H2SO4), including: (i) the oxidation of primary alcohols to form aldehydes and carboxylic acids; the control of the oxidation product using different reaction conditions, ( ...
... Candidates should be able to: (a) describe the oxidation of alcohols (see also unit F322: 2.2.1.f) using Cr2O72-/H+ (ie K2Cr2O7/H2SO4), including: (i) the oxidation of primary alcohols to form aldehydes and carboxylic acids; the control of the oxidation product using different reaction conditions, ( ...
ppt
... The Wittig reaction gives C=C in a defined location, based on the location of the carbonyl group (C=O) CH3 ...
... The Wittig reaction gives C=C in a defined location, based on the location of the carbonyl group (C=O) CH3 ...
Ring-closing metathesis

Ring-closing metathesis, or RCM, is a widely used variation of olefin metathesis in organic chemistry for the synthesis of various unsaturated rings via the intramolecular metathesis of two terminal alkenes, which forms the cycloalkene as the E- or Z- isomers and volatile ethylene.The most commonly synthesized ring sizes are between 5-7 atoms; however, reported syntheses include 45- up to 90- membered macroheterocycles. These reactions are metal-catalyzed and proceed through a metallacyclobutane intermediate. It was first published by Dider Villemin in 1980 describing the synthesis of an Exaltolide precursor, and later become popularized by Robert H. Grubbs and Richard R. Schrock, who shared the Nobel Prize in Chemistry, along with Yves Chauvin, in 2005 for their combined work in olefin metathesis. RCM is a favorite among organic chemists due to its synthetic utility in the formation of rings, which were previously difficult to access efficiently, and broad substrate scope. Since the only major by-product is ethylene, these reactions may also be considered atom economic, an increasingly important concern in the development of green chemistry.There are several reviews published on ring-closing metathesis.