Olefin hydroformylation catalysis with RuCl2(DMSO)4.
... biphasic organic solvent/ water systems. The substrates tried were 1-hexene, cyclohexene, 2-methyl-2-pentene, 2,3dimethyl-2-butene; binary mixtures and synthetic naphtha and real naphtha. The activity is better for linear olefins compared with substituted olefins. Key words: hydroformylation reactio ...
... biphasic organic solvent/ water systems. The substrates tried were 1-hexene, cyclohexene, 2-methyl-2-pentene, 2,3dimethyl-2-butene; binary mixtures and synthetic naphtha and real naphtha. The activity is better for linear olefins compared with substituted olefins. Key words: hydroformylation reactio ...
Practice Exam 4 - BioChemWeb.net
... NMR signals (ppm): 2.0 (3H, doublet); 6.1 (1H, doublet of doublets); 6.9 (1H, broad multiplet); 9.5 (1H, doublet) 1H ...
... NMR signals (ppm): 2.0 (3H, doublet); 6.1 (1H, doublet of doublets); 6.9 (1H, broad multiplet); 9.5 (1H, doublet) 1H ...
l - CMatthews
... 5. An electrochemical cell consists of silver and copper electrodes immersed in 1.0 mol solutions of silver nitrate and copper (II) nitrate respectively. Potassium chloride solution is in the salt bridge. a) Write the half cell reactions that occur at each electrode. b) Write the cell reaction and c ...
... 5. An electrochemical cell consists of silver and copper electrodes immersed in 1.0 mol solutions of silver nitrate and copper (II) nitrate respectively. Potassium chloride solution is in the salt bridge. a) Write the half cell reactions that occur at each electrode. b) Write the cell reaction and c ...
review sheet
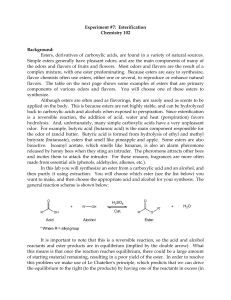
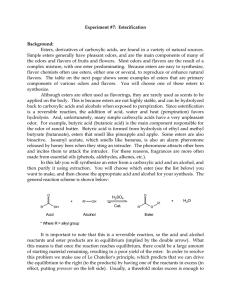
... Know the following reaction mechanisms: Acetal formation (acid catalyzed) Fisher esterification (acid catalyzed) Ester hydrolysis (acid catalyzed) Nucleophilic acyl substitution (up-down-out) Example: acid chloride + alcohol to give ester Ester reaction with Grignard reagents Questions that may be o ...
... Know the following reaction mechanisms: Acetal formation (acid catalyzed) Fisher esterification (acid catalyzed) Ester hydrolysis (acid catalyzed) Nucleophilic acyl substitution (up-down-out) Example: acid chloride + alcohol to give ester Ester reaction with Grignard reagents Questions that may be o ...
The Formation of 2,2,4-Trimethyl-2,3-dihydro-1H-1,5
... Reactions of o-phenylenediamine with a dicarboxylic acid can produce several different products depending on the specific conditions [1]. In the presence of cyclization agents such as hydrochloric acid or polyphosphoric acid, these reactions have been reported to give benzimidazoles [2,3]. This is a ...
... Reactions of o-phenylenediamine with a dicarboxylic acid can produce several different products depending on the specific conditions [1]. In the presence of cyclization agents such as hydrochloric acid or polyphosphoric acid, these reactions have been reported to give benzimidazoles [2,3]. This is a ...
SORAN UNIVERSITY
... A. Knowledge and understanding: Students will have an understanding through study different subjects in organic and the biochemistry, like (nomenclatures, reactions, physical and chemicals properties) for the most organic compounds. On the other hand the students can be establishing the products whi ...
... A. Knowledge and understanding: Students will have an understanding through study different subjects in organic and the biochemistry, like (nomenclatures, reactions, physical and chemicals properties) for the most organic compounds. On the other hand the students can be establishing the products whi ...
CHEM 109A CLAS Alkenes and Reactions of
... Electrophilic Addition Rxns In general: π e-s of double bond attracted to electrophile (alkene is a Nuc) & get addition of electrophile to 1 vinyl C and Nuc adds to the other vinyl C. Can be used to synthesize alkyl halides (EX above), alcohols, ethers, epoxides and alkanes. Addition of Hydrogen Hal ...
... Electrophilic Addition Rxns In general: π e-s of double bond attracted to electrophile (alkene is a Nuc) & get addition of electrophile to 1 vinyl C and Nuc adds to the other vinyl C. Can be used to synthesize alkyl halides (EX above), alcohols, ethers, epoxides and alkanes. Addition of Hydrogen Hal ...
No Slide Title - Cobalt
... The role of the electronic and the steric effects has been investigated, by considering the simplified (generic) models and the examples of the real catalysts. An energy decomposition of the contributions to the binding energies has been performed in order to understand the origin of the differences ...
... The role of the electronic and the steric effects has been investigated, by considering the simplified (generic) models and the examples of the real catalysts. An energy decomposition of the contributions to the binding energies has been performed in order to understand the origin of the differences ...
15 - MSU Chemistry
... The starting material in the first reaction has a plane of symmetry so it is achiral: the stereochemistry shows only which diastereoisomer we have. Attack by the amine nucleophile at either end ...
... The starting material in the first reaction has a plane of symmetry so it is achiral: the stereochemistry shows only which diastereoisomer we have. Attack by the amine nucleophile at either end ...
Ring-closing metathesis

Ring-closing metathesis, or RCM, is a widely used variation of olefin metathesis in organic chemistry for the synthesis of various unsaturated rings via the intramolecular metathesis of two terminal alkenes, which forms the cycloalkene as the E- or Z- isomers and volatile ethylene.The most commonly synthesized ring sizes are between 5-7 atoms; however, reported syntheses include 45- up to 90- membered macroheterocycles. These reactions are metal-catalyzed and proceed through a metallacyclobutane intermediate. It was first published by Dider Villemin in 1980 describing the synthesis of an Exaltolide precursor, and later become popularized by Robert H. Grubbs and Richard R. Schrock, who shared the Nobel Prize in Chemistry, along with Yves Chauvin, in 2005 for their combined work in olefin metathesis. RCM is a favorite among organic chemists due to its synthetic utility in the formation of rings, which were previously difficult to access efficiently, and broad substrate scope. Since the only major by-product is ethylene, these reactions may also be considered atom economic, an increasingly important concern in the development of green chemistry.There are several reviews published on ring-closing metathesis.