PHASE TRANSFER CATALYSIS IN PHARMACEUTICAL
... system, induced by chiral TAA salts was not obvious. The chiral catalyst, according to commonly accepted mechanistic picture of its action, exerts only ionic and hydrophobic interaction with reacting substrate. Thus its effect, differentiating energies of the two diastereomeric transition states lea ...
... system, induced by chiral TAA salts was not obvious. The chiral catalyst, according to commonly accepted mechanistic picture of its action, exerts only ionic and hydrophobic interaction with reacting substrate. Thus its effect, differentiating energies of the two diastereomeric transition states lea ...
CH 12-3 Power Point
... •The 3-step series of reactions includes the unique reaction of an alkyl halide (R-X) with magnesium metal (Mg) to form a C-Mg “organic-metallic” bond: ...
... •The 3-step series of reactions includes the unique reaction of an alkyl halide (R-X) with magnesium metal (Mg) to form a C-Mg “organic-metallic” bond: ...
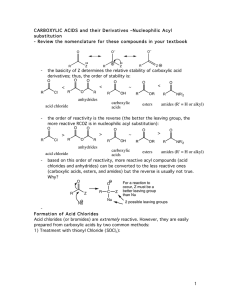
carboxylic acids esters amides (R
... to prepare in bulk from dirt-cheap acetic acid, and so it is often used in place of acetyl chloride (MeCOCl). Second, cyclic anhydrides are fairly easily formed by heating molecules that have two carboxylic acid functions in close proximity to high temperatures (a dehydration reaction). A couple of ...
... to prepare in bulk from dirt-cheap acetic acid, and so it is often used in place of acetyl chloride (MeCOCl). Second, cyclic anhydrides are fairly easily formed by heating molecules that have two carboxylic acid functions in close proximity to high temperatures (a dehydration reaction). A couple of ...
Scientific abstract
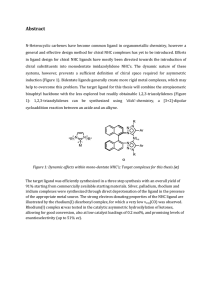
... chiral substituents into monodentate imidazolylidene NHC’s. The dynamic nature of these systems, however, prevents a sufficient definition of chiral space required for asymmetric induction (Figure 1). Bidentate ligands generally create more rigid metal complexes, which may help to overcome this prob ...
... chiral substituents into monodentate imidazolylidene NHC’s. The dynamic nature of these systems, however, prevents a sufficient definition of chiral space required for asymmetric induction (Figure 1). Bidentate ligands generally create more rigid metal complexes, which may help to overcome this prob ...
Aqueous oxidation of alcohols catalyzed by artificial
... in DMF (31 ll), streptavidin (0.08 lmol of the tetrameric protein) in a mixture of water (500 ll) and acetone (150 ll), acetophenone was obtained with 81% conversion after 90 h at room temperature (Table 1, entry 1). Extraction of the reaction mixture with pentane allows to isolate the product devoi ...
... in DMF (31 ll), streptavidin (0.08 lmol of the tetrameric protein) in a mixture of water (500 ll) and acetone (150 ll), acetophenone was obtained with 81% conversion after 90 h at room temperature (Table 1, entry 1). Extraction of the reaction mixture with pentane allows to isolate the product devoi ...
Acid-Catalyzed Dehydration of Alcohols
... as a layer on the surface of the acid-alcohol mixture (the density of the alkene is less than one); the gas can then be collected over water or the liquid layer can be removed by simple distillation to give the final alkene product. In alcohols where there are more than two kinks of -hydrogens, the ...
... as a layer on the surface of the acid-alcohol mixture (the density of the alkene is less than one); the gas can then be collected over water or the liquid layer can be removed by simple distillation to give the final alkene product. In alcohols where there are more than two kinks of -hydrogens, the ...
Islamic University of Gaza
... ) 10- Unsymmetrical ethers are generally prepared by acidic dehydration of alcohols. ...
... ) 10- Unsymmetrical ethers are generally prepared by acidic dehydration of alcohols. ...
ch15
... Organic Compounds and the Atomic Properties of Carbon 15.1 The Special Nature of Carbon and the Characteristics of Organic Molecules 15.2 The Structures and Classes of Hydrocarbons 15.3 Some Important Classes of Organic Reactions 15.4 Properties and Reactivities of Common Functional Groups 15.5 The ...
... Organic Compounds and the Atomic Properties of Carbon 15.1 The Special Nature of Carbon and the Characteristics of Organic Molecules 15.2 The Structures and Classes of Hydrocarbons 15.3 Some Important Classes of Organic Reactions 15.4 Properties and Reactivities of Common Functional Groups 15.5 The ...
11 - MSU Chemistry
... Cyclic acetals are more stable than non-‐cyclic ones as we explain on p. 228 of the textbook. Hydrolysis needs more vigorous conditions. Thioacetals are much harder to hydrolyse because sulfides are ...
... Cyclic acetals are more stable than non-‐cyclic ones as we explain on p. 228 of the textbook. Hydrolysis needs more vigorous conditions. Thioacetals are much harder to hydrolyse because sulfides are ...
Types of reactions you know:
... Google “McMurrray Organic Chem” and it should be the first hit. I will also have a link on the SI website to the website. The “organic interactive” problems in each chapter are extremely helpful. Even if you don’t use this to study for the final, it is a good quick and easy review after your o-chem- ...
... Google “McMurrray Organic Chem” and it should be the first hit. I will also have a link on the SI website to the website. The “organic interactive” problems in each chapter are extremely helpful. Even if you don’t use this to study for the final, it is a good quick and easy review after your o-chem- ...
Microsoft Word
... This thesis describes the synthesis and characterization of selected early and late transition metal complexes and their usefulness as catalysts for the polymerization of ethylene. Polymerization of ethylene was studied using copper(II) complexes based on -diimine, bis(oxazoline) and bis(benzimidaz ...
... This thesis describes the synthesis and characterization of selected early and late transition metal complexes and their usefulness as catalysts for the polymerization of ethylene. Polymerization of ethylene was studied using copper(II) complexes based on -diimine, bis(oxazoline) and bis(benzimidaz ...
Ring-closing metathesis

Ring-closing metathesis, or RCM, is a widely used variation of olefin metathesis in organic chemistry for the synthesis of various unsaturated rings via the intramolecular metathesis of two terminal alkenes, which forms the cycloalkene as the E- or Z- isomers and volatile ethylene.The most commonly synthesized ring sizes are between 5-7 atoms; however, reported syntheses include 45- up to 90- membered macroheterocycles. These reactions are metal-catalyzed and proceed through a metallacyclobutane intermediate. It was first published by Dider Villemin in 1980 describing the synthesis of an Exaltolide precursor, and later become popularized by Robert H. Grubbs and Richard R. Schrock, who shared the Nobel Prize in Chemistry, along with Yves Chauvin, in 2005 for their combined work in olefin metathesis. RCM is a favorite among organic chemists due to its synthetic utility in the formation of rings, which were previously difficult to access efficiently, and broad substrate scope. Since the only major by-product is ethylene, these reactions may also be considered atom economic, an increasingly important concern in the development of green chemistry.There are several reviews published on ring-closing metathesis.