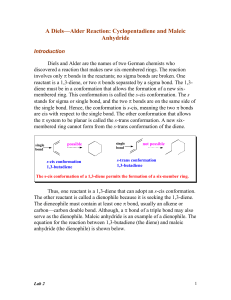
Atom efficiency and catalysis in organic synthesis
... (Fig. 6) using a combination of RuCl2(Ph3P)3 and TEMPO (2,2',6,6'-tetramethylpiperidine-N-oxyl) as the catalyst [31]. The method is effective for the conversion of a broad range of primary, secondary, and allylic alcohols to the corresponding aldehydes or ketones. Many other examples of selective ca ...
... (Fig. 6) using a combination of RuCl2(Ph3P)3 and TEMPO (2,2',6,6'-tetramethylpiperidine-N-oxyl) as the catalyst [31]. The method is effective for the conversion of a broad range of primary, secondary, and allylic alcohols to the corresponding aldehydes or ketones. Many other examples of selective ca ...
Organic Chemistry - Snow College | It's SNOWing
... • Uses a better leaving group • Conditions are not as extreme • Phosphorous oxychloride and pyridine • No rearrangements • Mildly basic conditions favor E2 ...
... • Uses a better leaving group • Conditions are not as extreme • Phosphorous oxychloride and pyridine • No rearrangements • Mildly basic conditions favor E2 ...
Synthesis of Aliphatic Nitro Compounds1i2 A simple new
... was present (DMSO and sodium nitrite dried as described above) was allowed to proceed for 8 hr. On working up as usual an 11% yield (5.3 g.) of 2-nitrooctane (b.p. 62'/2 mm.; n: 1.4281) and an 11% yield (5.3 g.) of 2-octyl nitrite (b.p. 35'/3 mm.; n'," 1.4088)were isolated. I n addition, there was o ...
... was present (DMSO and sodium nitrite dried as described above) was allowed to proceed for 8 hr. On working up as usual an 11% yield (5.3 g.) of 2-nitrooctane (b.p. 62'/2 mm.; n: 1.4281) and an 11% yield (5.3 g.) of 2-octyl nitrite (b.p. 35'/3 mm.; n'," 1.4088)were isolated. I n addition, there was o ...
Formation of N-Methylated Cyclic Ligand Systems from Unusual
... coordinatively “innocent” solvents.3 We have earlier reported that, in the presence of acetonitrile/TMNO, Fe3E2(CO)9 (E ) S, Se, Te) complexes are converted into Fe2E2(CO)6, which can react with coordinatively unsaturated organometallic fragments to form mixed-metal clusters. Thus, the reaction of F ...
... coordinatively “innocent” solvents.3 We have earlier reported that, in the presence of acetonitrile/TMNO, Fe3E2(CO)9 (E ) S, Se, Te) complexes are converted into Fe2E2(CO)6, which can react with coordinatively unsaturated organometallic fragments to form mixed-metal clusters. Thus, the reaction of F ...
SOLVENT-FREE SYNTHESIS OF CHALCONE BY ALDOL
... Chalcones represent a group of compounds with interesting biological activities that are formed from an aldol condensation between a benzaldehyde and an acetophenone in the presence of NaOH as a catalyst. Although traditionally synthesized using aqueous sodium hydroxide in organic solvents, in this ...
... Chalcones represent a group of compounds with interesting biological activities that are formed from an aldol condensation between a benzaldehyde and an acetophenone in the presence of NaOH as a catalyst. Although traditionally synthesized using aqueous sodium hydroxide in organic solvents, in this ...
Final Exam, Chem 111 2012 Study Guide (labs)
... b. From measurements, rank half-cells in order of increasing half-cell reduction potential. c. Convert measured half-cell reduction potentials to values relative to S.H.E. d. Predict new cell potentials from measurements of half-cell potentials. ...
... b. From measurements, rank half-cells in order of increasing half-cell reduction potential. c. Convert measured half-cell reduction potentials to values relative to S.H.E. d. Predict new cell potentials from measurements of half-cell potentials. ...
Substitution reactions of carbonyl compounds at the α
... stabilize enolate charges, and lead to a large variety of different compounds. However, you’ll only get decarboxylation if you have at least one group that can be hydrolyzed to an acid, which is separated from another carbonyl group by one carbon. For example: ...
... stabilize enolate charges, and lead to a large variety of different compounds. However, you’ll only get decarboxylation if you have at least one group that can be hydrolyzed to an acid, which is separated from another carbonyl group by one carbon. For example: ...
Science24-UnitA-Section3.1-3.2
... Types of Reactions When you study for school, do you put things that are similar together? Do you look for patterns when you try solving a mathematics problem? Similarly, in chemistry, you can group chemical reactions together according to particular patterns in which the reactions occur. The most c ...
... Types of Reactions When you study for school, do you put things that are similar together? Do you look for patterns when you try solving a mathematics problem? Similarly, in chemistry, you can group chemical reactions together according to particular patterns in which the reactions occur. The most c ...
CARBONYL COMPOUNDS
... and HCHO (MR = 30) and put these compounds in order with respect to increasing boiling point. C2H6 CH3OH HCHO Methanal is a gas, other important carbonyl compounds are …………………... Early members are soluble in water due to ……………….. ………………. between hydrogen from water and oxygen from the carboxylic gro ...
... and HCHO (MR = 30) and put these compounds in order with respect to increasing boiling point. C2H6 CH3OH HCHO Methanal is a gas, other important carbonyl compounds are …………………... Early members are soluble in water due to ……………….. ………………. between hydrogen from water and oxygen from the carboxylic gro ...
BSc-Chemistry-II
... Mechanisms of nucleophilic substitution in nitroarenes and their reductions in acidic, neutral and alkaline media. Picric acid. Alkyl and Aryl amines : Reactivity, structure and nomenclature of amines, physical properties. Stereochemistry of amines. Separation of a mixture of primary, secondary and ...
... Mechanisms of nucleophilic substitution in nitroarenes and their reductions in acidic, neutral and alkaline media. Picric acid. Alkyl and Aryl amines : Reactivity, structure and nomenclature of amines, physical properties. Stereochemistry of amines. Separation of a mixture of primary, secondary and ...
Ring-closing metathesis

Ring-closing metathesis, or RCM, is a widely used variation of olefin metathesis in organic chemistry for the synthesis of various unsaturated rings via the intramolecular metathesis of two terminal alkenes, which forms the cycloalkene as the E- or Z- isomers and volatile ethylene.The most commonly synthesized ring sizes are between 5-7 atoms; however, reported syntheses include 45- up to 90- membered macroheterocycles. These reactions are metal-catalyzed and proceed through a metallacyclobutane intermediate. It was first published by Dider Villemin in 1980 describing the synthesis of an Exaltolide precursor, and later become popularized by Robert H. Grubbs and Richard R. Schrock, who shared the Nobel Prize in Chemistry, along with Yves Chauvin, in 2005 for their combined work in olefin metathesis. RCM is a favorite among organic chemists due to its synthetic utility in the formation of rings, which were previously difficult to access efficiently, and broad substrate scope. Since the only major by-product is ethylene, these reactions may also be considered atom economic, an increasingly important concern in the development of green chemistry.There are several reviews published on ring-closing metathesis.