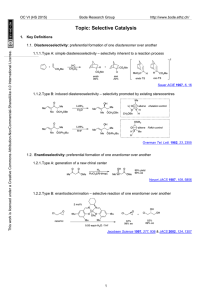
Changing counterion can switch the preference for selective 1,2
... Another example of a selectivity-controlled reaction of alkenes is hydroformylation via the use of scaffolding ligands. These bind covalently and reversibly to the substrate, leading to a temporarily intramolecular transformation that can lead to dramatically improved and reversed selectivity with s ...
... Another example of a selectivity-controlled reaction of alkenes is hydroformylation via the use of scaffolding ligands. These bind covalently and reversibly to the substrate, leading to a temporarily intramolecular transformation that can lead to dramatically improved and reversed selectivity with s ...
lecture 11 catalysis_hydrogenation of alkenes
... The protons of a dihydrogen ligand are known to be more acidic than those of free H2, and many H2 complexes can be deprotonated by NEt3. ...
... The protons of a dihydrogen ligand are known to be more acidic than those of free H2, and many H2 complexes can be deprotonated by NEt3. ...
Alcohols, Phenols, and Ethers
... (1) Alcohols have higher boiling points than alkanes of similar molecular mass because of hydrogen bonding. (2) Ethylene glycol contains two carbon atoms and two hydroxyl groups. (3) Primary and secondary alcohols give the same type of product when subjected to mild oxidizing agents. a) All three st ...
... (1) Alcohols have higher boiling points than alkanes of similar molecular mass because of hydrogen bonding. (2) Ethylene glycol contains two carbon atoms and two hydroxyl groups. (3) Primary and secondary alcohols give the same type of product when subjected to mild oxidizing agents. a) All three st ...
Hein and Arena - faculty at Chemeketa
... electrons between carbon and magnesium are shifted away from the electropositive Mg to form a strongly polar covalent bond. As a result the charge distribution in the Grignard reagent is such that the organic group (R) is partially negative and the –MgX group is partially positive. This charge distr ...
... electrons between carbon and magnesium are shifted away from the electropositive Mg to form a strongly polar covalent bond. As a result the charge distribution in the Grignard reagent is such that the organic group (R) is partially negative and the –MgX group is partially positive. This charge distr ...
Chapter Twenty-four
... • A functional group is a group of atoms that is largely responsible for the chemical behavior of the parent molecule. • Most organic compounds are derived from a group of compounds known as hydrocarbons because they are made up of only hydrogen and carbon. • Carbon has the ability to form long chai ...
... • A functional group is a group of atoms that is largely responsible for the chemical behavior of the parent molecule. • Most organic compounds are derived from a group of compounds known as hydrocarbons because they are made up of only hydrogen and carbon. • Carbon has the ability to form long chai ...
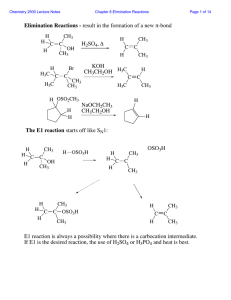
Four new mechanisms to learn: SN2 vs E2 and SN1 vs E1
... SN1 and E1 reactions are multistep reactions and also compete with one another. Both of these reactions begin with the same rate-limiting step of carbocation formation from an R-X compound. Carbocations (R+) are very reactive electron deficient carbon intermediates that typically follow one of three ...
... SN1 and E1 reactions are multistep reactions and also compete with one another. Both of these reactions begin with the same rate-limiting step of carbocation formation from an R-X compound. Carbocations (R+) are very reactive electron deficient carbon intermediates that typically follow one of three ...
Aminoketone Rearrangements. 11. The Rearrangement of Phenyl a
... When the aminoketone I I a was heated with methylamine and methanol, i t was partially converted into the unconjugated aminoketone IIIa, as shown by infrared analysis. These are the same conditions under which these aminoketones were formed in the original epoxyether reaction. This indicated that I ...
... When the aminoketone I I a was heated with methylamine and methanol, i t was partially converted into the unconjugated aminoketone IIIa, as shown by infrared analysis. These are the same conditions under which these aminoketones were formed in the original epoxyether reaction. This indicated that I ...
Improved Synthesis, Separation, Transition Metal Coordination and
... requirement for the degree of Doctor of Philosophy in The Department of Chemistry ...
... requirement for the degree of Doctor of Philosophy in The Department of Chemistry ...
Organic Chemistry
... • Step 2: attack of halide ion (a nucleophile) from the opposite side of the bromonium ion (an electrophile) opens the three-membered ring to give the product Br ...
... • Step 2: attack of halide ion (a nucleophile) from the opposite side of the bromonium ion (an electrophile) opens the three-membered ring to give the product Br ...
Grignard Reagents brochure
... react with a broad range of electrophilic substrates. The reactions with aldehydes, ketones27, esters, acids49 and acid chlorides is one of the most useful reaction in organic chemistry for the formation of C-C-bonds50,51,52. The reaction has a very broad scope, and the Grignard reagent can be aliph ...
... react with a broad range of electrophilic substrates. The reactions with aldehydes, ketones27, esters, acids49 and acid chlorides is one of the most useful reaction in organic chemistry for the formation of C-C-bonds50,51,52. The reaction has a very broad scope, and the Grignard reagent can be aliph ...
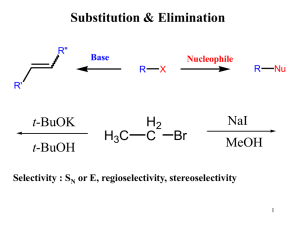
幻灯片 1
... H and X are on the same side of the molecule, and anti periplanar geometry, in which the H and the X are on opposite sides of the molecule. Of the two choices, anti periplanar geometry is energetically preferred because it allows the substituents on the two carbons to adopt a staggered relationship, ...
... H and X are on the same side of the molecule, and anti periplanar geometry, in which the H and the X are on opposite sides of the molecule. Of the two choices, anti periplanar geometry is energetically preferred because it allows the substituents on the two carbons to adopt a staggered relationship, ...
Q4) How the following conversions can be carried out?
... corresponding salts and dissolve in aq. Na2CO3 solution. Q5. (i) Why is the Dipole moment of methanol higher than that of phenol? (ii) . Explain why phenols do not undergo substitution of the –OH group like alcohols. ANS. (i) Due to electron withdrawing effect of phenyl group, the C—O bond in phenol ...
... corresponding salts and dissolve in aq. Na2CO3 solution. Q5. (i) Why is the Dipole moment of methanol higher than that of phenol? (ii) . Explain why phenols do not undergo substitution of the –OH group like alcohols. ANS. (i) Due to electron withdrawing effect of phenyl group, the C—O bond in phenol ...
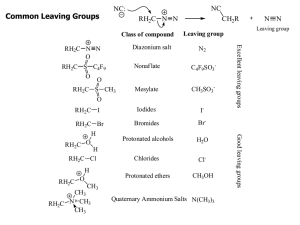
Common Leaving Groups
... •To favour E1 over SN1 for alcohols, use an acid with a non-nucleophilic conjugate base (H2SO4, H3PO4). To favour SN1 over E1, use a good nucleophile. •To favour E2 over SN2, use a strong, bulky non-nucleophilic base. To favour SN2 over E2, use good nucleophiles that are relatively weak bases. •It i ...
... •To favour E1 over SN1 for alcohols, use an acid with a non-nucleophilic conjugate base (H2SO4, H3PO4). To favour SN1 over E1, use a good nucleophile. •To favour E2 over SN2, use a strong, bulky non-nucleophilic base. To favour SN2 over E2, use good nucleophiles that are relatively weak bases. •It i ...
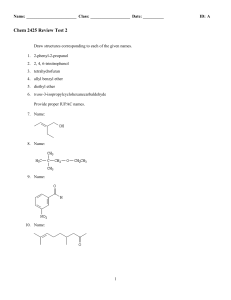
Chem 2423-Test 2 - HCC Learning Web
... Oxygen does not affect the base formula. A hydrogen is added to the base formula for each halogen and subtracted for each nitrogen so the base formula for diazepam is C16H12. The saturated 16 carbon compound would have 34 hydrogens so the number of degrees of unsaturation for diazepam is: (34 − 12) ...
... Oxygen does not affect the base formula. A hydrogen is added to the base formula for each halogen and subtracted for each nitrogen so the base formula for diazepam is C16H12. The saturated 16 carbon compound would have 34 hydrogens so the number of degrees of unsaturation for diazepam is: (34 − 12) ...
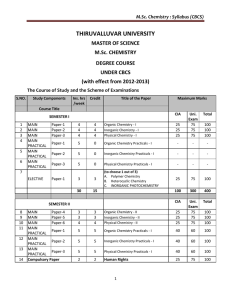
M.Sc. Chemistry : Syllabus (CBCS)
... Fischer projections. Molecules with more than one asymmetric center (restricted to five carbons).e.g. Erythro and threo compounds. Asymmetric synthesis.Cram’s rule. UNIT-II: CONFORMATIONAL ANALYSIS Conformational analysis ofdisubstituted cyclohexane and their stereo chemical features (geometric and ...
... Fischer projections. Molecules with more than one asymmetric center (restricted to five carbons).e.g. Erythro and threo compounds. Asymmetric synthesis.Cram’s rule. UNIT-II: CONFORMATIONAL ANALYSIS Conformational analysis ofdisubstituted cyclohexane and their stereo chemical features (geometric and ...
Slide 1
... Ethers resemble hydrocarbons in their resistance to chemical reaction. • They do not react with oxidizing agents such as potassium dichromate. • They do not react with reducing agents such as H2 in the presence of a transition metal catalyst. • They are not affected by most acids or bases at moderat ...
... Ethers resemble hydrocarbons in their resistance to chemical reaction. • They do not react with oxidizing agents such as potassium dichromate. • They do not react with reducing agents such as H2 in the presence of a transition metal catalyst. • They are not affected by most acids or bases at moderat ...
Alcohols, Phenols, and Ethers
... As molecular weight increases for alcohols, they become more “alkanelike”. Long chain alcohols are less soluble in water and more soluble in nonpolar solvents, like benzene. ...
... As molecular weight increases for alcohols, they become more “alkanelike”. Long chain alcohols are less soluble in water and more soluble in nonpolar solvents, like benzene. ...
Document
... • The E1 dehydration of 20 and 30 alcohols with acid gives clean elimination products without any by-products formed from an SN1 reaction. • Clean elimination takes place because the reaction mixture contains no good nucleophile to react with the intermediate carbocation, so no competing SN1 reactio ...
... • The E1 dehydration of 20 and 30 alcohols with acid gives clean elimination products without any by-products formed from an SN1 reaction. • Clean elimination takes place because the reaction mixture contains no good nucleophile to react with the intermediate carbocation, so no competing SN1 reactio ...
Preparation of ethers
... the reactants in a liquid phase. symmetrical or unsymmetrical in nature. Speci?cally The process of this invention may be effected in any speaking the preparation of these ethers is accomplished 65 suitable manner and may comprise either a batch or con tinuous type operation. For example, when a bat ...
... the reactants in a liquid phase. symmetrical or unsymmetrical in nature. Speci?cally The process of this invention may be effected in any speaking the preparation of these ethers is accomplished 65 suitable manner and may comprise either a batch or con tinuous type operation. For example, when a bat ...
Synthesis of Nitrogen-, Oxygen- and Sulphur
... after hydrolysis under mild conditions gave the target compound 2a in 35% yield. With this protocol in hand, we examined the scope and limitations of this method using a series of alkylcarbamate reactants (5b-5e). The results indicated that the reaction is effective for a range of aliphatic and arom ...
... after hydrolysis under mild conditions gave the target compound 2a in 35% yield. With this protocol in hand, we examined the scope and limitations of this method using a series of alkylcarbamate reactants (5b-5e). The results indicated that the reaction is effective for a range of aliphatic and arom ...
Chem 2425-Test 2 Review
... Cyanohydrins are important intermediates in the synthesis of α-hydroxycarboxylic acids from ketones and aldehydes. The nitrile functional group can be hydrolyzed by aqueous acid to yield a carboxylic acid. Nitriles can also be hydrolyzed to carboxylic acids using aqueous base. Unfortunately, when a ...
... Cyanohydrins are important intermediates in the synthesis of α-hydroxycarboxylic acids from ketones and aldehydes. The nitrile functional group can be hydrolyzed by aqueous acid to yield a carboxylic acid. Nitriles can also be hydrolyzed to carboxylic acids using aqueous base. Unfortunately, when a ...
Forward
... So, too, are many other alcohols. All alcohols of four carbons or fewer, as well as most of the five- and six-carbon alcohols and many higher alcohols, are commercially available at low cost. Some occur naturally; others are the products of efficient syntheses. Figure 15.1 presents the structures of ...
... So, too, are many other alcohols. All alcohols of four carbons or fewer, as well as most of the five- and six-carbon alcohols and many higher alcohols, are commercially available at low cost. Some occur naturally; others are the products of efficient syntheses. Figure 15.1 presents the structures of ...
Elias James Corey

Elias James ""E.J."" Corey (born July 12, 1928) is an American organic chemist. In 1990, he won the Nobel Prize in Chemistry ""for his development of the theory and methodology of organic synthesis"", specifically retrosynthetic analysis. Regarded by many as one of the greatest living chemists, he has developed numerous synthetic reagents, methodologies and total syntheses and has advanced the science of organic synthesis considerably.