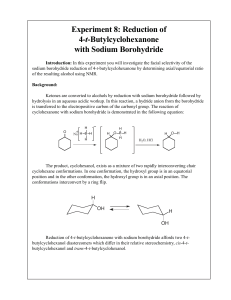
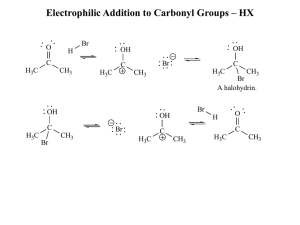
answers_ch03
... Due to these problems, it is necessary to protect the amino group of one amino acid and the carboxylic acid of the other before a coupling reaction is attempted. It is also important to have coupling reagents (e.g. dicyclohexylcarbodiimide) in order to achieve milder, non-racemising conditions. ...
... Due to these problems, it is necessary to protect the amino group of one amino acid and the carboxylic acid of the other before a coupling reaction is attempted. It is also important to have coupling reagents (e.g. dicyclohexylcarbodiimide) in order to achieve milder, non-racemising conditions. ...
Class: 11 Subject: Chemistry Topic: Equilibrium No. of
... 10. Two moles of nitrogen and two moles of hydrogen are taken in a closed vessel of a five litre capacity and suitable conditions are provided for the reaction. When equilibrium is reached it is found that half a mole of nitrogen is used up. The equilibrium concentration of ammonia is A. 0.2 B. 0.4 ...
... 10. Two moles of nitrogen and two moles of hydrogen are taken in a closed vessel of a five litre capacity and suitable conditions are provided for the reaction. When equilibrium is reached it is found that half a mole of nitrogen is used up. The equilibrium concentration of ammonia is A. 0.2 B. 0.4 ...
Notetakers
... Under certain conditions, ethene can also undergo addition reactions with itself to form a long chain polymer containing many thousands (typically 40,000 to 800,000) of carbon atoms. ...
... Under certain conditions, ethene can also undergo addition reactions with itself to form a long chain polymer containing many thousands (typically 40,000 to 800,000) of carbon atoms. ...
Document
... are many such orbitals (an infinite number) but the focusing always on the electrons that are most important for the chemistry. These are: the highest occupied molecule orbital or HOMO and the lowest un-occupied molecular orbital or LUMO. The HOMO is the highest energy MO that has any electrons in i ...
... are many such orbitals (an infinite number) but the focusing always on the electrons that are most important for the chemistry. These are: the highest occupied molecule orbital or HOMO and the lowest un-occupied molecular orbital or LUMO. The HOMO is the highest energy MO that has any electrons in i ...
ch11 - alcohols and ethers
... 1. Ethers by Intermolecular Dehydration of Alcohol l Primary alcohols can dehydrate to ethers è This reaction occurs at lower temperature than the competing dehydration to an alkene è This method generally does not work with secondary or tertiary alcohols because elimination competes strongly ...
... 1. Ethers by Intermolecular Dehydration of Alcohol l Primary alcohols can dehydrate to ethers è This reaction occurs at lower temperature than the competing dehydration to an alkene è This method generally does not work with secondary or tertiary alcohols because elimination competes strongly ...
CHEM 212B, Organic Chemistry - City College of San Francisco
... A continuation of CHEM 212A. The second semester of a one-year course in organic chemistry for students who major in chemistry, biochemistry, and other chemistry-intensive sciences. IV. MAJOR LEARNING OUTCOMES Upon completion of this course a student will be able to: A. Name and draw the structures ...
... A continuation of CHEM 212A. The second semester of a one-year course in organic chemistry for students who major in chemistry, biochemistry, and other chemistry-intensive sciences. IV. MAJOR LEARNING OUTCOMES Upon completion of this course a student will be able to: A. Name and draw the structures ...
Summary of Reactions Which Will Appear on Exams
... (this is the only reaction you will need to balance; for all others only the organic product(s) required) ...
... (this is the only reaction you will need to balance; for all others only the organic product(s) required) ...
Chapter 4
... • All functional groups contain a heteroatom, a bond or both. • These features create electrophilic sites and nucleophilic sites in a molecule. • Electron-rich sites (nucleophiles) react with electron poor sites (electrophiles). • An electronegative heteroatom like N, O, or X makes a carbon atom e ...
... • All functional groups contain a heteroatom, a bond or both. • These features create electrophilic sites and nucleophilic sites in a molecule. • Electron-rich sites (nucleophiles) react with electron poor sites (electrophiles). • An electronegative heteroatom like N, O, or X makes a carbon atom e ...
Unit 3: Chemical Kinetics
... This reaction does not occur in a single step, however, but rather through these two steps: Step 1: 2 NO → N2O2 Step 2: N2O2 + O2 → 2 NO2 Notice that if you add these two reactions together, you end up with the overall reaction: Step 1: 2 NO → N2O2 Step 2: N2O2 + O2 → 2 NO2 Overall: 2 NO(g) + O2 → ...
... This reaction does not occur in a single step, however, but rather through these two steps: Step 1: 2 NO → N2O2 Step 2: N2O2 + O2 → 2 NO2 Notice that if you add these two reactions together, you end up with the overall reaction: Step 1: 2 NO → N2O2 Step 2: N2O2 + O2 → 2 NO2 Overall: 2 NO(g) + O2 → ...
Module 2 Asymmetric Carbon-Carbon Bond Forming Reactions
... styrene, in the case of Mo-based system, RORCM product is formed with 92% ee. The substrate used for the Ru-catalyzed ROCM process, proceed polymerization in the presence of Mo-catalyst instead of ROCM process. Scheme 7 shows the comparison of the Ru-catalyzed ROCM of norbornenes. The catalysts 7 an ...
... styrene, in the case of Mo-based system, RORCM product is formed with 92% ee. The substrate used for the Ru-catalyzed ROCM process, proceed polymerization in the presence of Mo-catalyst instead of ROCM process. Scheme 7 shows the comparison of the Ru-catalyzed ROCM of norbornenes. The catalysts 7 an ...
Chemkin-Pro
... combustion system design. Because this condition Accurate emissions prediction with chemistry is dictated by real chemistry and fluid dynamics, Combustor design simulation requires resolution of complex geometry, turbulent flow patterns, heat the reduced chemistry intrinsic to most CFD tools cannot ...
... combustion system design. Because this condition Accurate emissions prediction with chemistry is dictated by real chemistry and fluid dynamics, Combustor design simulation requires resolution of complex geometry, turbulent flow patterns, heat the reduced chemistry intrinsic to most CFD tools cannot ...
Reactions of Alcohols
... • Ethanol oxidizes to acetaldehyde, then acetic acid, a normal metabolite. • Methanol oxidizes to formaldehyde, then formic acid, more toxic than methanol. • Ethylene glycol oxidizes to oxalic acid, toxic. • Treatment for poisoning is excess ethanol. ...
... • Ethanol oxidizes to acetaldehyde, then acetic acid, a normal metabolite. • Methanol oxidizes to formaldehyde, then formic acid, more toxic than methanol. • Ethylene glycol oxidizes to oxalic acid, toxic. • Treatment for poisoning is excess ethanol. ...
Organic Compounds
... • LPG = Propane and Butane • Petrol = Mixture of hydrocarbons (5 – 10 C alkanes) • Kerosene = 10 – 14 C alkanes used for heating fuel and Jet fuel ...
... • LPG = Propane and Butane • Petrol = Mixture of hydrocarbons (5 – 10 C alkanes) • Kerosene = 10 – 14 C alkanes used for heating fuel and Jet fuel ...
Reaction Rate Reading Packet
... 3. Surface area is the measure of how much area of an object is exposed. For the same mass, many small particles have a greater total surface area than one large particle. For example, steel wool has a larger surface area than a block of steel of the same mass. This allows oxygen molecules to collid ...
... 3. Surface area is the measure of how much area of an object is exposed. For the same mass, many small particles have a greater total surface area than one large particle. For example, steel wool has a larger surface area than a block of steel of the same mass. This allows oxygen molecules to collid ...
Asymmetric induction

Asymmetric induction (also enantioinduction) in stereochemistry describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis.Asymmetric induction was introduced by Hermann Emil Fischer based on his work on carbohydrates. Several types of induction exist.Internal asymmetric induction makes use of a chiral center bound to the reactive center through a covalent bond and remains so during the reaction. The starting material is often derived from chiral pool synthesis. In relayed asymmetric induction the chiral information is introduced in a separate step and removed again in a separate chemical reaction. Special synthons are called chiral auxiliaries. In external asymmetric induction chiral information is introduced in the transition state through a catalyst of chiral ligand. This method of asymmetric synthesis is economically most desirable.