Organic Chemistry | Topic Notes
... • -ane is replaced by -anal. • Remember; methanal, propanal, ethanal and butanal or 2-methylpropanal. • The C = O group or Carbonyl group is highly polar. This means that compounds containing a carbonyl group cannot for hydrogen bonds. • The boiling points of Aldehydes are higher than the correspond ...
... • -ane is replaced by -anal. • Remember; methanal, propanal, ethanal and butanal or 2-methylpropanal. • The C = O group or Carbonyl group is highly polar. This means that compounds containing a carbonyl group cannot for hydrogen bonds. • The boiling points of Aldehydes are higher than the correspond ...
Organic and Biochemistry
... When two or more substituents are present, list them in alphabetical order. • When there are two or more of the same substituent, the number of that type of substituent is indicated by a prefix: (i.e., “dimethyl” indicates two methyl group substituents). ...
... When two or more substituents are present, list them in alphabetical order. • When there are two or more of the same substituent, the number of that type of substituent is indicated by a prefix: (i.e., “dimethyl” indicates two methyl group substituents). ...
Welcome to Class 7
... keto groups and hydroxyl groups. In aqueous solutions, most monosaccharides occur as cyclic structures. They result from hemiacetal or hemiketal formation between aldehyde or keto groups and hydroxyl groups on the same molecule. The reaction is freely reversible. ...
... keto groups and hydroxyl groups. In aqueous solutions, most monosaccharides occur as cyclic structures. They result from hemiacetal or hemiketal formation between aldehyde or keto groups and hydroxyl groups on the same molecule. The reaction is freely reversible. ...
AlCl3 in modern chemistry of polyfluoroarenes
... well [4,5]. Recently for acid-catalyzed reactions of different type compounds along with traditional mineral acids and Lewis acids new type catalysts were started to use, for example solid acids and solid super acids [6 and references], metal complexes (Hf, Sc, Ga, Bi, Yb) with -N(SO2 C8 F17)2 and - ...
... well [4,5]. Recently for acid-catalyzed reactions of different type compounds along with traditional mineral acids and Lewis acids new type catalysts were started to use, for example solid acids and solid super acids [6 and references], metal complexes (Hf, Sc, Ga, Bi, Yb) with -N(SO2 C8 F17)2 and - ...
Structure and Properties of Organic Molecules Reading: Wade
... hexane (C6H14) and H2O are immiscible. V. Classes of organic compounds A. Hydrocarbons: compounds consisting only of carbon and hydrogen. a. Alkanes: examples: CH4, CH3CH2CH2CH3, cyclopentane, cyclohexane. Alkyl groups are an alkane portion of a molecule: -CH3 (methyl), -CH2CH3 (ethyl), -CH2CH2CH3 ( ...
... hexane (C6H14) and H2O are immiscible. V. Classes of organic compounds A. Hydrocarbons: compounds consisting only of carbon and hydrogen. a. Alkanes: examples: CH4, CH3CH2CH2CH3, cyclopentane, cyclohexane. Alkyl groups are an alkane portion of a molecule: -CH3 (methyl), -CH2CH3 (ethyl), -CH2CH2CH3 ( ...
handout alkenes from alcohols
... This procedure has been adapted from the microscale procedure described in the third edition of Macroscale and Microscale Organic Experiments by Kenneth L. Williamson (Houghton Mifflin, Boston, 1999). ...
... This procedure has been adapted from the microscale procedure described in the third edition of Macroscale and Microscale Organic Experiments by Kenneth L. Williamson (Houghton Mifflin, Boston, 1999). ...
Exam 3 Key - Chemistry
... ketone C=O str. would be about: a) 1725 cm-1 b) 1785 cm-1 c) 1560 cm-1 d) 1705 cm-1 e) 1715 cm-1 10. (3) If the position for an aldehyde C=O str. in the IR spectrum is 1715 cm-1 then an -unsaturated aldehyde C=O str. would be about: a) 1725 cm-1 b) 1785 cm-1 c) 1560 cm-1 d) 1700 cm-1 e) 1715 cm-1 ...
... ketone C=O str. would be about: a) 1725 cm-1 b) 1785 cm-1 c) 1560 cm-1 d) 1705 cm-1 e) 1715 cm-1 10. (3) If the position for an aldehyde C=O str. in the IR spectrum is 1715 cm-1 then an -unsaturated aldehyde C=O str. would be about: a) 1725 cm-1 b) 1785 cm-1 c) 1560 cm-1 d) 1700 cm-1 e) 1715 cm-1 ...
2.9 database - DrBravoChemistry
... Give the name and draw the graphical formula of an alkene that is an isomer of but-1-ene and that has a different carbon skeleton. Name ................................................................................................................ Graphical formula ...
... Give the name and draw the graphical formula of an alkene that is an isomer of but-1-ene and that has a different carbon skeleton. Name ................................................................................................................ Graphical formula ...
Here is the Original File - University of New Hampshire
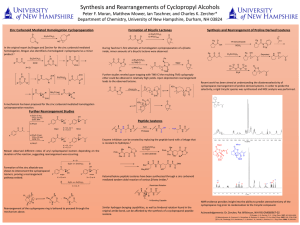
... Further studies reveled upon trapping with TMS-Cl the resulting TMS cycloproply ether could be obtained in relatively high yields. Upon deprotection rearrangement leads to the observed lactone. ...
... Further studies reveled upon trapping with TMS-Cl the resulting TMS cycloproply ether could be obtained in relatively high yields. Upon deprotection rearrangement leads to the observed lactone. ...
chemistry important question i
... (i) Nickel (ii) Germanium Mention the principle behind each of them. Explain the mechanism of acid catalysed hydration of an alkene to form corresponding alcohol. ...
... (i) Nickel (ii) Germanium Mention the principle behind each of them. Explain the mechanism of acid catalysed hydration of an alkene to form corresponding alcohol. ...
Alkane Alkyl groups are represented by the R
... group and two hydrogens attached to a nitrogen atom. Primary amines can be shown in text as: RNH2 Primary amines are basic functions that can be protonated to the ...
... group and two hydrogens attached to a nitrogen atom. Primary amines can be shown in text as: RNH2 Primary amines are basic functions that can be protonated to the ...
PREPARATION OF ORGANOLITHIUM COMPOUNDS - GCG-42
... Carbon-metal bonds are polar bonds that can be represented by a resonance hybrid of covalent and ionic structures. C-Li are strong bases and react with acids, even weak acids like water and alcohols. ...
... Carbon-metal bonds are polar bonds that can be represented by a resonance hybrid of covalent and ionic structures. C-Li are strong bases and react with acids, even weak acids like water and alcohols. ...
Gas and Vapor Phase Explosions
... 3. Find the spark coil and turn it on by rotating the knob on its bottom clockwise. You will hear it begin to make a sparking sound. CAUTION: Do not touch the tip as you will get a shock from it! 4. Touch one of the screws in the polyethylene bottle with the tip of the spark coil and stand back! 5. ...
... 3. Find the spark coil and turn it on by rotating the knob on its bottom clockwise. You will hear it begin to make a sparking sound. CAUTION: Do not touch the tip as you will get a shock from it! 4. Touch one of the screws in the polyethylene bottle with the tip of the spark coil and stand back! 5. ...
09 Stoichiometry WS Stoichiometry WS
... 4. What mass of sulfuric acid, H2SO4, is required to react with 1.27 g of potassium hydroxide? The products of this reaction are potassium sulfate and water. 5. Ammonium hydrogen phosphate, (NH4)2HPO4, a common fertilizer, is made from reacting phosphoric acid, H3PO4, with ammonia. a. Write the equa ...
... 4. What mass of sulfuric acid, H2SO4, is required to react with 1.27 g of potassium hydroxide? The products of this reaction are potassium sulfate and water. 5. Ammonium hydrogen phosphate, (NH4)2HPO4, a common fertilizer, is made from reacting phosphoric acid, H3PO4, with ammonia. a. Write the equa ...
Chapter 17 Allylic and Benzylic Reactivity
... substituents outweigh their resonance effects. Consequently, compound (3) reacts more slowly. The nitro group exerts no resonance effect in the carbocation intermediates derived from compounds (1) and (4); the question is then whether its polar effect is stronger from the meta or para position. As i ...
... substituents outweigh their resonance effects. Consequently, compound (3) reacts more slowly. The nitro group exerts no resonance effect in the carbocation intermediates derived from compounds (1) and (4); the question is then whether its polar effect is stronger from the meta or para position. As i ...
Screening - Entrance
... 34. strongly acidic solutions, aniline becomes more reactive towards electrophilic reagents. ...
... 34. strongly acidic solutions, aniline becomes more reactive towards electrophilic reagents. ...
Chapter 12: Aldehydes, Ketones and Carboxylic acids
... Reactions of aldehydes and ketones: Aldehydes are generally more reactive than ketones in nucleophilic addition reactions due to steric and electronic reasons (or inductive effect). Electronic Effect: Relative reactivities of aldehydes and ketones in nucleophilic addition reactions is due the positi ...
... Reactions of aldehydes and ketones: Aldehydes are generally more reactive than ketones in nucleophilic addition reactions due to steric and electronic reasons (or inductive effect). Electronic Effect: Relative reactivities of aldehydes and ketones in nucleophilic addition reactions is due the positi ...
Asymmetric induction

Asymmetric induction (also enantioinduction) in stereochemistry describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis.Asymmetric induction was introduced by Hermann Emil Fischer based on his work on carbohydrates. Several types of induction exist.Internal asymmetric induction makes use of a chiral center bound to the reactive center through a covalent bond and remains so during the reaction. The starting material is often derived from chiral pool synthesis. In relayed asymmetric induction the chiral information is introduced in a separate step and removed again in a separate chemical reaction. Special synthons are called chiral auxiliaries. In external asymmetric induction chiral information is introduced in the transition state through a catalyst of chiral ligand. This method of asymmetric synthesis is economically most desirable.