Double Bond
... sterically more difficult than abstracting a more exposed methyl hydrogen when a hindered base is used. The transition state leading to the more stable product is increased in energy by steric interference with the bulky base. ...
... sterically more difficult than abstracting a more exposed methyl hydrogen when a hindered base is used. The transition state leading to the more stable product is increased in energy by steric interference with the bulky base. ...
handout alkenes from alcohols
... This procedure has been adapted from the microscale procedure described in the third edition of Macroscale and Microscale Organic Experiments by Kenneth L. Williamson (Houghton Mifflin, Boston, 1999). ...
... This procedure has been adapted from the microscale procedure described in the third edition of Macroscale and Microscale Organic Experiments by Kenneth L. Williamson (Houghton Mifflin, Boston, 1999). ...
Organic Chemistry (HL) Revision Questions
... If the conditions of the reaction in (b) are changed so that a hot solution of sodium hydroxide in ethanol is used then a different reaction occurs. The reaction with 2-bromo-2-methylbutane gives two different organic products. State the type of reaction taking place and suggest the identity (name o ...
... If the conditions of the reaction in (b) are changed so that a hot solution of sodium hydroxide in ethanol is used then a different reaction occurs. The reaction with 2-bromo-2-methylbutane gives two different organic products. State the type of reaction taking place and suggest the identity (name o ...
NC Exam Questions - Rosshall Academy
... 17. When vegetable oils are hydrolysed, mixtures of fatty acids are obtained. The fatty acids can be classified by their degree of unsaturation. The table below shows the composition of each of the mixtures of fatty acids obtained when palm oil and olive oil were ...
... 17. When vegetable oils are hydrolysed, mixtures of fatty acids are obtained. The fatty acids can be classified by their degree of unsaturation. The table below shows the composition of each of the mixtures of fatty acids obtained when palm oil and olive oil were ...
File - the prayas tutorial
... Ans. Because Grignard reagents have a very strong affinity for H+ ions. In presence of water, they abstract H+ ions from water and form alkanes. To prevent this, they should be prepared under anhydrous conditions. Q. 7. Haloalkanes react with KCN to form alkyl cyanides as major product while AgCN fo ...
... Ans. Because Grignard reagents have a very strong affinity for H+ ions. In presence of water, they abstract H+ ions from water and form alkanes. To prevent this, they should be prepared under anhydrous conditions. Q. 7. Haloalkanes react with KCN to form alkyl cyanides as major product while AgCN fo ...
File - Mr Weng`s IB Chemistry
... formula, which differ from each other by a common structural unit. • Structural formulas can be represented in full and condensed format. • Structural isomers are compounds with the same molecular formula but different arrangements of atoms. • Functional groups are the reactive parts of molecules. • ...
... formula, which differ from each other by a common structural unit. • Structural formulas can be represented in full and condensed format. • Structural isomers are compounds with the same molecular formula but different arrangements of atoms. • Functional groups are the reactive parts of molecules. • ...
PERIODIC TABLE
... 40- When (C8H16) is burned in oxygen atmosphere, we obtain (CO2) and (H2O) according to the following equation: a C8H16 + b O2 → c CO2 + d H2O In a balanced equation, the factors a, b, c, and d have the values: a- (a = 1, b = 1, c = 1, d = 1) b- (a = 1, b = 12, c = 8, d = 16) c- (a = 1, b = 12, c = ...
... 40- When (C8H16) is burned in oxygen atmosphere, we obtain (CO2) and (H2O) according to the following equation: a C8H16 + b O2 → c CO2 + d H2O In a balanced equation, the factors a, b, c, and d have the values: a- (a = 1, b = 1, c = 1, d = 1) b- (a = 1, b = 12, c = 8, d = 16) c- (a = 1, b = 12, c = ...
Topic 11 Organic Chemistry
... the following pairs of organic substances, whose boiling points are given: • ethane (184 K) and butane (273 K); • ethane ( 184 K) and bromoethane (311 K); • bromoethane (311 K) and ethanol (352 K). ...
... the following pairs of organic substances, whose boiling points are given: • ethane (184 K) and butane (273 K); • ethane ( 184 K) and bromoethane (311 K); • bromoethane (311 K) and ethanol (352 K). ...
CfE Higher Chemistry Homework Unit 2: Natures Chemistry
... Which line in the table correctly compares the properties of vanillin and zingerone? ...
... Which line in the table correctly compares the properties of vanillin and zingerone? ...
Review Questions
... needed to raise the temperature of the kettle and its contents to 80.0 oC? 5. If you have 60 kg of water heated from 15oC to 700C, how much energy is required? 6. Using standard enthalpies of formation calculate the enthalpy change for: 4 NH3 (g) + 5 O2 (g) 4 NO(g) + 6 H2O (g) ...
... needed to raise the temperature of the kettle and its contents to 80.0 oC? 5. If you have 60 kg of water heated from 15oC to 700C, how much energy is required? 6. Using standard enthalpies of formation calculate the enthalpy change for: 4 NH3 (g) + 5 O2 (g) 4 NO(g) + 6 H2O (g) ...
32 GRIGNARD REACTION Alkyl halides can react with magnesium
... GRIGNARD REACTION Alkyl halides can react with magnesium to give a very versatile synthetic reagent, the Grignard reagent, for which Victor Grignard received the Nobel Prize in 1912. The Grignard reagent, RMgX, is one of the most useful reagents known. Although the carbon magnesium bond is covalent, ...
... GRIGNARD REACTION Alkyl halides can react with magnesium to give a very versatile synthetic reagent, the Grignard reagent, for which Victor Grignard received the Nobel Prize in 1912. The Grignard reagent, RMgX, is one of the most useful reagents known. Although the carbon magnesium bond is covalent, ...
Chapter 10 for 302
... There cannot be any acidic protons in the solvent, as the Grignard is such a strong base. There cannot be any pi bonds in the solvent as those are sites of reactivity that the Grignard will attack. From here on, I will use Grignard to refer to both Grignard reagents and organolithiums, as they ...
... There cannot be any acidic protons in the solvent, as the Grignard is such a strong base. There cannot be any pi bonds in the solvent as those are sites of reactivity that the Grignard will attack. From here on, I will use Grignard to refer to both Grignard reagents and organolithiums, as they ...
Electophilic Aromatic Substituion
... The product is formed by loss of a proton, which is replaced by bromine FeBr3 is added as a catalyst to polarize the bromine reagent In the first step the electrons act as a nucleophile toward Br2 (in a complex with FeBr3) This forms a cationic addition intermediate from benzene and ...
... The product is formed by loss of a proton, which is replaced by bromine FeBr3 is added as a catalyst to polarize the bromine reagent In the first step the electrons act as a nucleophile toward Br2 (in a complex with FeBr3) This forms a cationic addition intermediate from benzene and ...
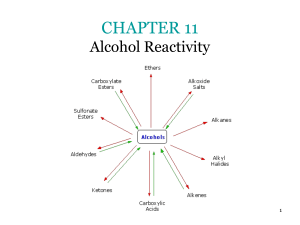
CHAPTER 9 Further Reactions of Alcohols and the Chemistry of
... Conversion of Alcohols to Alkyl sulfonates creates a good leaving group for subsequent displacement by an anionic nucleophile ...
... Conversion of Alcohols to Alkyl sulfonates creates a good leaving group for subsequent displacement by an anionic nucleophile ...
Chemical Equations
... • MS-PS1-5. I can explain the conservation of mass through a model of chemical reactions. • MS-PS1-3 I can gather information to describe the origins and impacts of synthetic material ...
... • MS-PS1-5. I can explain the conservation of mass through a model of chemical reactions. • MS-PS1-3 I can gather information to describe the origins and impacts of synthetic material ...
Why is sugar sweet?
... relatively non-polar part between the hydrogen bond donor and acceptor. In sugars, the non-polar part consists of the C-H bonds adjacent to the OH groups. Sweetness varies with structure. In the table below, table sugar (sucrose) is given an arbitrary sweetness value of 1.0 and other sweet substance ...
... relatively non-polar part between the hydrogen bond donor and acceptor. In sugars, the non-polar part consists of the C-H bonds adjacent to the OH groups. Sweetness varies with structure. In the table below, table sugar (sucrose) is given an arbitrary sweetness value of 1.0 and other sweet substance ...
OCR A Level Chemistry B (Salters) Multiple Choice Questions Quiz
... We’d like to know your view on the resources we produce. By clicking on ‘Like’ or ‘Dislike’ you can help us to ensure that our resources work for you. When the email template pops up please add additional comments if you wish and then just click ‘Send’. Thank you. If you do not currently offer this ...
... We’d like to know your view on the resources we produce. By clicking on ‘Like’ or ‘Dislike’ you can help us to ensure that our resources work for you. When the email template pops up please add additional comments if you wish and then just click ‘Send’. Thank you. If you do not currently offer this ...
Asymmetric induction

Asymmetric induction (also enantioinduction) in stereochemistry describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis.Asymmetric induction was introduced by Hermann Emil Fischer based on his work on carbohydrates. Several types of induction exist.Internal asymmetric induction makes use of a chiral center bound to the reactive center through a covalent bond and remains so during the reaction. The starting material is often derived from chiral pool synthesis. In relayed asymmetric induction the chiral information is introduced in a separate step and removed again in a separate chemical reaction. Special synthons are called chiral auxiliaries. In external asymmetric induction chiral information is introduced in the transition state through a catalyst of chiral ligand. This method of asymmetric synthesis is economically most desirable.