Pseudoasymmetry as a Means for Distinguishing Meso
... have shown that it is possible to differentiate between cis2,s-dimethylpyrrolidine (a meso diastereomer) and truns2,s-dimethylpyrrolidine (a dl diastereomer), and related a,o-disubstituted cyclic amines, by conversion to their N benzyl derivatives and examination of their N M R spectra.19%20 The ben ...
... have shown that it is possible to differentiate between cis2,s-dimethylpyrrolidine (a meso diastereomer) and truns2,s-dimethylpyrrolidine (a dl diastereomer), and related a,o-disubstituted cyclic amines, by conversion to their N benzyl derivatives and examination of their N M R spectra.19%20 The ben ...
Aminoketone Rearrangements. 11. The Rearrangement of Phenyl a
... of C14-tracer studies that migration results, even in the absence of conditions favorable to rearrangement, such as release of steric strain. The starting ketone recovered after treatment of CI4-labeled p-anisylanisoin with base showed a statistical distribution of C14label. The rearrangements consi ...
... of C14-tracer studies that migration results, even in the absence of conditions favorable to rearrangement, such as release of steric strain. The starting ketone recovered after treatment of CI4-labeled p-anisylanisoin with base showed a statistical distribution of C14label. The rearrangements consi ...
10.4 Alcohols - SCIS Teachers
... •The shorter chain alcohols such as methanol and ethanol are similar to water, in general they •have higher boiling points than hydrocarbons but lower than water •dissolve in water to some degree •are more polar than hydrocarbons but less polar than water ...
... •The shorter chain alcohols such as methanol and ethanol are similar to water, in general they •have higher boiling points than hydrocarbons but lower than water •dissolve in water to some degree •are more polar than hydrocarbons but less polar than water ...
Appendix
... of Cu wire with HNO3, diluting to volume in a 500-mL volumetric flask, and then diluting a 1-mL portion of this stock solution to volume in a 250-mL volumetric flask. To calculate the overall uncertainty we included the uncertainty in the sample's mass and the uncertainty of the volumetric glassware ...
... of Cu wire with HNO3, diluting to volume in a 500-mL volumetric flask, and then diluting a 1-mL portion of this stock solution to volume in a 250-mL volumetric flask. To calculate the overall uncertainty we included the uncertainty in the sample's mass and the uncertainty of the volumetric glassware ...
english,
... may be realized by reverse hydrolysis (condensation) or transfer reaction (transglycosylation).8 Transglycosylation type reactions often employ glycosyl donors with good leaving groups, such as glycosyl fluorides14 or p-nitrophenyl glycosides.15 Vulfson et al.16 reported that cellobiose gave alkyl b ...
... may be realized by reverse hydrolysis (condensation) or transfer reaction (transglycosylation).8 Transglycosylation type reactions often employ glycosyl donors with good leaving groups, such as glycosyl fluorides14 or p-nitrophenyl glycosides.15 Vulfson et al.16 reported that cellobiose gave alkyl b ...
Functional Groups
... carbon: four covalent bonds and no unshared pairs of electrons hydrogen: one covalent bond and no unshared pairs of electrons nitrogen: three covalent bonds and one unshared pair of electrons oxygen: two covalent bonds and two unshared pairs of electrons a halogen: one covalent bond and three unshar ...
... carbon: four covalent bonds and no unshared pairs of electrons hydrogen: one covalent bond and no unshared pairs of electrons nitrogen: three covalent bonds and one unshared pair of electrons oxygen: two covalent bonds and two unshared pairs of electrons a halogen: one covalent bond and three unshar ...
+ ∂ - CHEM171 – Lecture Series Seven : 2012/05
... A hydrocarbon of unknown structure has the molecular formula C8H10. On catalytic hydrogenation over the Lindlar catalyst, 1 equivalent of H2 is absorbed. On hydrogenation over a Pd/C catalyst 3 equivalents of H2 react. Draw a structure for the hydrocarbon that fits the data. ...
... A hydrocarbon of unknown structure has the molecular formula C8H10. On catalytic hydrogenation over the Lindlar catalyst, 1 equivalent of H2 is absorbed. On hydrogenation over a Pd/C catalyst 3 equivalents of H2 react. Draw a structure for the hydrocarbon that fits the data. ...
Chapter-16B
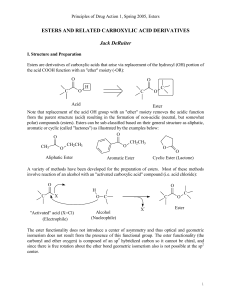
... There are two major differences between acidcatalyzed and base-promoted ester hydrolysis 1. for acid-catalyzed hydrolysis, acid is required in only catalytic amounts; for base-promoted hydrolysis, base is required in equimolar amounts 2. hydrolysis of an ester in aqueous acid is reversible; base-pro ...
... There are two major differences between acidcatalyzed and base-promoted ester hydrolysis 1. for acid-catalyzed hydrolysis, acid is required in only catalytic amounts; for base-promoted hydrolysis, base is required in equimolar amounts 2. hydrolysis of an ester in aqueous acid is reversible; base-pro ...
INTERPRETATION OF INFRARED SPECTRA Hydrocarbons
... The carbonyl (C=O) stretch is one of the most distinctive bands in the IR spectrum. It can be found at 1640-1820 cm-1 and is generally a very strong peak. Functional Group ...
... The carbonyl (C=O) stretch is one of the most distinctive bands in the IR spectrum. It can be found at 1640-1820 cm-1 and is generally a very strong peak. Functional Group ...
Discovery and Exploitation of AZADO: The Highly Active Catalyst for
... dition to its fascinating “green” features, TEMPO oxidation is distinguished from other oxidation methods by its ability to conduct the selective oxidation of primary alcohols in the presence of secondary alcohols.13–16) The rationale behind such a feature is its reaction mechanism and its structure ...
... dition to its fascinating “green” features, TEMPO oxidation is distinguished from other oxidation methods by its ability to conduct the selective oxidation of primary alcohols in the presence of secondary alcohols.13–16) The rationale behind such a feature is its reaction mechanism and its structure ...
Unit-8-Alcohols-Aldehydes
... • Markovnikov’s Rule can be used to predict which of the two products is predicted to be the major product. -The hydrogen from the water in a hydration reaction is added to the double-bonded carbon atom that originally carried the most hydrogen atoms. ...
... • Markovnikov’s Rule can be used to predict which of the two products is predicted to be the major product. -The hydrogen from the water in a hydration reaction is added to the double-bonded carbon atom that originally carried the most hydrogen atoms. ...
Mineralization of Drugs in Aqueous Medium by Advanced Oxidation
... electro-Fenton process involves the enhancement of the oxidizing power of the above electrolytic system by adding small amounts of a catalyst like Fe2+, which reacts with electrogenerated H2O2 to yield •OH in solution from Fenton’s reaction (1). The most popular electro-Fenton method is the so-calle ...
... electro-Fenton process involves the enhancement of the oxidizing power of the above electrolytic system by adding small amounts of a catalyst like Fe2+, which reacts with electrogenerated H2O2 to yield •OH in solution from Fenton’s reaction (1). The most popular electro-Fenton method is the so-calle ...
4. a-Monohalo Ethers in Protection Chemistry
... (4) (Dimethyl-t-butylsilyloxy)methyl chloride (SOMCl) The usual was to cleave SEM ethers is by treatment with fluoride ions. It has, however, been reported that fluoride cleavage of SEM ethers has failed.[Kozikowski, 1987] In such cases and for the protection of sterically hindered alcohols a relat ...
... (4) (Dimethyl-t-butylsilyloxy)methyl chloride (SOMCl) The usual was to cleave SEM ethers is by treatment with fluoride ions. It has, however, been reported that fluoride cleavage of SEM ethers has failed.[Kozikowski, 1987] In such cases and for the protection of sterically hindered alcohols a relat ...
Chapter 14
... At first glance this looks impossible. How are we to break one of the unactivated C-C single bonds in cyclohexane to make a 1,6-diol with two extra carbons! So, rather than looking at the starting material, look at the target molecule and look at the first disconnection shown by the wavy line. If we ...
... At first glance this looks impossible. How are we to break one of the unactivated C-C single bonds in cyclohexane to make a 1,6-diol with two extra carbons! So, rather than looking at the starting material, look at the target molecule and look at the first disconnection shown by the wavy line. If we ...
Petasis reaction

The Petasis reaction (alternatively called the Petasis borono–Mannich (PBM) reaction) is the chemical reaction of an amine, aldehyde, and vinyl- or aryl-boronic acid to form substituted amines.Reported in 1993 by Nicos Petasis as a practical method towards the synthesis of a geometrically pure antifungal agent, naftifine, the Petasis reaction can be described as a variation of the Mannich reaction. Rather than generating an enolate to form the substituted amine product, in the Petasis reaction, the vinyl group of the organoboronic acid serves as the nucleophile. In comparison to other methods of generating allyl amines, the Petasis reaction tolerates a multifunctional scaffold, with a variety of amines and organoboronic acids as potential starting materials. Additionally, the reaction does not require anhydrous or inert conditions. As a mild, selective synthesis, the Petasis reaction is useful in generating α-amino acids, and is utilized in combinatorial chemistry and drug discovery.