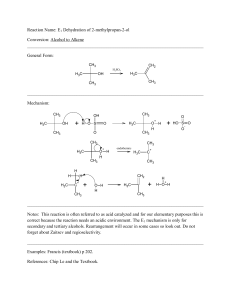
1990-Spring-Exam-2-student
... will make a new octane booster, methyl t-butyl ether (MTBE). OUTLINE an acceptable route to prepare MTBE from available materials in a minimum # of steps consistent with high yield. (Structure of MTBE "For Sale" for 3 pts.) ...
... will make a new octane booster, methyl t-butyl ether (MTBE). OUTLINE an acceptable route to prepare MTBE from available materials in a minimum # of steps consistent with high yield. (Structure of MTBE "For Sale" for 3 pts.) ...
Chapter 15 Lipids Lipids Types of Lipids Classes of Lipids Fatty
... The chemical reactions of triacylglycerols are similar to those of alkenes and esters. ...
... The chemical reactions of triacylglycerols are similar to those of alkenes and esters. ...
Organic Chemistry/Fourth Edition: e-Text
... The mechanisms of all the reactions cited in Table 20.2 are similar to the mechanism of hydrolysis of an acyl chloride outlined in Figure 20.3. They differ with respect to the nucleophile that attacks the carbonyl group. In the first stage of the mechanism, water undergoes nucleophilic addition to t ...
... The mechanisms of all the reactions cited in Table 20.2 are similar to the mechanism of hydrolysis of an acyl chloride outlined in Figure 20.3. They differ with respect to the nucleophile that attacks the carbonyl group. In the first stage of the mechanism, water undergoes nucleophilic addition to t ...
Chapter 10
... The proton adds in the first step to generate the carbocation adjacent to the oxygen due to resonance from the lone pair stabilizing the cation, thus directing the regiochemistry ...
... The proton adds in the first step to generate the carbocation adjacent to the oxygen due to resonance from the lone pair stabilizing the cation, thus directing the regiochemistry ...
Handout VI
... Trivially, aldehydes are usually named after the acids they form on oxidation. The –ic in the name of the acid is then replaced by aldehyde to get the name of the aldehyde. ...
... Trivially, aldehydes are usually named after the acids they form on oxidation. The –ic in the name of the acid is then replaced by aldehyde to get the name of the aldehyde. ...
Chapter 3
... • in primary, secondary, tertiary alcohols, the OH group is bonded to a primary, secondary, tertiary carbon • primary, secondary, tertiary carbons are connected to one, two, three alkyl groups ...
... • in primary, secondary, tertiary alcohols, the OH group is bonded to a primary, secondary, tertiary carbon • primary, secondary, tertiary carbons are connected to one, two, three alkyl groups ...
The reaction pathways of hydrogen peroxide in
... enthalpy, entropy and free energy of the transition states of the formation and breakdown of the intermediate have been calculated. The metal-catalyzed pathway of hydrogen peroxide is dealing with the effect of hydroxyl radicals created by the Fenton reaction and their potential to oxidize the disul ...
... enthalpy, entropy and free energy of the transition states of the formation and breakdown of the intermediate have been calculated. The metal-catalyzed pathway of hydrogen peroxide is dealing with the effect of hydroxyl radicals created by the Fenton reaction and their potential to oxidize the disul ...
Chapter 12 Alcohols, Phenols, Ethers, Aldehydes, and Ketones
... alcohols and ketones are reduced to 2° alcohols Alcohols ...
... alcohols and ketones are reduced to 2° alcohols Alcohols ...
HMDS - Sigma
... is readily lost from the transition state during reaction, but possesses sufficient chemical stability in combination with the alkyl silyl group to allow long term storage of the derivatizing agent for use as required. As the formation of the transition state is reversible, the derivatization will o ...
... is readily lost from the transition state during reaction, but possesses sufficient chemical stability in combination with the alkyl silyl group to allow long term storage of the derivatizing agent for use as required. As the formation of the transition state is reversible, the derivatization will o ...
A convenient method for the preparation of oxazaborolidine catalyst
... purpose.2–4 Even though the parent catalyst H-CBS 4, prepared in situ using a,a-diphenylpyrrolidinemethanol and BH3–THF, has been found to give good results in asymmetric reductions, the corresponding B-methyl derivative is preferred. However, this reduction method is not entirely satisfactory, part ...
... purpose.2–4 Even though the parent catalyst H-CBS 4, prepared in situ using a,a-diphenylpyrrolidinemethanol and BH3–THF, has been found to give good results in asymmetric reductions, the corresponding B-methyl derivative is preferred. However, this reduction method is not entirely satisfactory, part ...
BSA + TMCS + TMSI - Sigma
... readily lost from the transition state during reaction, but possesses sufficient chemical stability in combination with the alkyl silyl group to allow long term storage of the derivatizing agent for use as required. As the formation of the transition state is reversible, the derivatization will only ...
... readily lost from the transition state during reaction, but possesses sufficient chemical stability in combination with the alkyl silyl group to allow long term storage of the derivatizing agent for use as required. As the formation of the transition state is reversible, the derivatization will only ...
Lecture - Ch 18
... different from that obtained by reaction of the trans isomer • Solution: – Epoxidation, in this case, is a syn addition of oxygen to a double bond – Original bond stereochemistry is retained; product is a meso compound CHE2202, Chapter 18 Learn, 27 ...
... different from that obtained by reaction of the trans isomer • Solution: – Epoxidation, in this case, is a syn addition of oxygen to a double bond – Original bond stereochemistry is retained; product is a meso compound CHE2202, Chapter 18 Learn, 27 ...
Chemistry 209 - Experiment 3, Fall 2002
... As in the previous experiment, you should make a note of as many characteristics of the substances to be tested as possible. Always note the color and physical form of each compound you work with in the lab. As you perform the different tests, try to observe and note any subtle differences between t ...
... As in the previous experiment, you should make a note of as many characteristics of the substances to be tested as possible. Always note the color and physical form of each compound you work with in the lab. As you perform the different tests, try to observe and note any subtle differences between t ...
Hydroxyl Compounds
... - Reason: a more highly substituted alkyl group inhibits solvation of the alkoxide ion and drives the dissociation equilibrium to the left. - For example: methanol is more acidic than t-butyl alcohol. • The present of electron-withdrawing atoms enhances the acidity of alcohols. - Reason: the electro ...
... - Reason: a more highly substituted alkyl group inhibits solvation of the alkoxide ion and drives the dissociation equilibrium to the left. - For example: methanol is more acidic than t-butyl alcohol. • The present of electron-withdrawing atoms enhances the acidity of alcohols. - Reason: the electro ...
13-Elimination Reactions
... isomer of an alkene. Regioselective elimination reactions, on the other hand, produce several different isomers, but give one isomer in greater quantity than the others. Whether a reaction is regiospecific or regioselective depends on whether the reaction prefers to eliminate only one particular β h ...
... isomer of an alkene. Regioselective elimination reactions, on the other hand, produce several different isomers, but give one isomer in greater quantity than the others. Whether a reaction is regiospecific or regioselective depends on whether the reaction prefers to eliminate only one particular β h ...
chapter 6-hydroxyl compounds
... - Reason: a more highly substituted alkyl group inhibits solvation of the alkoxide ion and drives the dissociation equilibrium to the left. - For example: methanol is more acidic than t-butyl alcohol. • The present of electron-withdrawing atoms enhances the acidity of alcohols. - Reason: the electro ...
... - Reason: a more highly substituted alkyl group inhibits solvation of the alkoxide ion and drives the dissociation equilibrium to the left. - For example: methanol is more acidic than t-butyl alcohol. • The present of electron-withdrawing atoms enhances the acidity of alcohols. - Reason: the electro ...
幻灯片 1
... Bonding in organic compounds at that time was thought to be of either the water type, as in alcohols, ROH, or of the radical type, as in ethers which would be given the formula RO. But Williamson, by his ether synthesis, showed that mixed ethers, with two different alkyl groups, could be prepared. ...
... Bonding in organic compounds at that time was thought to be of either the water type, as in alcohols, ROH, or of the radical type, as in ethers which would be given the formula RO. But Williamson, by his ether synthesis, showed that mixed ethers, with two different alkyl groups, could be prepared. ...
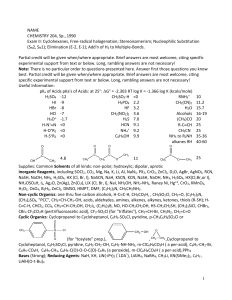
Chemistry - CBSE Guess
... 18. Half-life period of a radioactive element is 100 seconds. Calculate the disintegration constant and average life period. How much time will it take for 90% decay? 19. (a) Describe the structure and magnetic behaviour of [Ni(CN)4]2– ion on the basis of valence bond theory. (Atomic No. of Ni = 28) ...
... 18. Half-life period of a radioactive element is 100 seconds. Calculate the disintegration constant and average life period. How much time will it take for 90% decay? 19. (a) Describe the structure and magnetic behaviour of [Ni(CN)4]2– ion on the basis of valence bond theory. (Atomic No. of Ni = 28) ...
MHS Student Guide to Organic Chemistry
... Chemicals compounds that contain the element Carbon are known as organic compounds. “Organic” comes from the fact that until the mid 1800’s it was thought that these chemicals could only be derived from living plant or animal components. In 1828 Friedrich Woher converted the inorganic ammonium salt ...
... Chemicals compounds that contain the element Carbon are known as organic compounds. “Organic” comes from the fact that until the mid 1800’s it was thought that these chemicals could only be derived from living plant or animal components. In 1828 Friedrich Woher converted the inorganic ammonium salt ...
chemical reactions and stoichiometry chemical reactions and
... HCN. How much molecular oxygen will be required for this synthesis? Strategy: This problem looks complicated, so it is a good idea to apply the seven-step problem-solving method. 1. This is a stoichiometry problem (how much?), in which we are asked to find the mass of a reactant. 2. To visualize thi ...
... HCN. How much molecular oxygen will be required for this synthesis? Strategy: This problem looks complicated, so it is a good idea to apply the seven-step problem-solving method. 1. This is a stoichiometry problem (how much?), in which we are asked to find the mass of a reactant. 2. To visualize thi ...
Oxidation catalytic system and oxidation process using the same
... compound of the following formula (1) (e.g., N-hydroxyphthalimide), and a co-catalyst (except phospho vanadomolybdic acid) containing an element selected from the group consisting of Group 2A elements of the Periodic Table of Elements, transition metals (Group 3A to 7A elements, Group 8 elements, Gr ...
... compound of the following formula (1) (e.g., N-hydroxyphthalimide), and a co-catalyst (except phospho vanadomolybdic acid) containing an element selected from the group consisting of Group 2A elements of the Periodic Table of Elements, transition metals (Group 3A to 7A elements, Group 8 elements, Gr ...
Petasis reaction

The Petasis reaction (alternatively called the Petasis borono–Mannich (PBM) reaction) is the chemical reaction of an amine, aldehyde, and vinyl- or aryl-boronic acid to form substituted amines.Reported in 1993 by Nicos Petasis as a practical method towards the synthesis of a geometrically pure antifungal agent, naftifine, the Petasis reaction can be described as a variation of the Mannich reaction. Rather than generating an enolate to form the substituted amine product, in the Petasis reaction, the vinyl group of the organoboronic acid serves as the nucleophile. In comparison to other methods of generating allyl amines, the Petasis reaction tolerates a multifunctional scaffold, with a variety of amines and organoboronic acids as potential starting materials. Additionally, the reaction does not require anhydrous or inert conditions. As a mild, selective synthesis, the Petasis reaction is useful in generating α-amino acids, and is utilized in combinatorial chemistry and drug discovery.


















![Covalently Bonded Platinum(II) Complexes of [alpha]](http://s1.studyres.com/store/data/022412983_1-66c66ee18551a43164a79702fd995f95-300x300.png)