Kinetic modelling of the Maillard reaction between proteins and sugars
... 1.3 Sugar degradation ...
... 1.3 Sugar degradation ...
UNIT - I THE SOLID STATE KEY CONCEPTS
... between nearest metal atoms is 287pm (Ag= 107.87g mol-1, NA= 6.022 X 1023). 8. What is the distance between Na+ and Cl- ions in NaCl crystal if its density 2.165 g cm-3. NaCl crystallizes in FCC lattice. 9. Analysis shows that Nickel oxide has Ni 0.98 O 1.00 what fractions of nickel exist as Ni2+ io ...
... between nearest metal atoms is 287pm (Ag= 107.87g mol-1, NA= 6.022 X 1023). 8. What is the distance between Na+ and Cl- ions in NaCl crystal if its density 2.165 g cm-3. NaCl crystallizes in FCC lattice. 9. Analysis shows that Nickel oxide has Ni 0.98 O 1.00 what fractions of nickel exist as Ni2+ io ...
Esterification and Esters
... esterification reaction mixture drives the equilibrium in favor of the ester product (39). Binary azeotropes may be formed between the alcohol and water, the alcohol and ester, and the ester and water. Ternary azeotropes involving the alcohol, ester, and water are also possible. In general, the tern ...
... esterification reaction mixture drives the equilibrium in favor of the ester product (39). Binary azeotropes may be formed between the alcohol and water, the alcohol and ester, and the ester and water. Ternary azeotropes involving the alcohol, ester, and water are also possible. In general, the tern ...
Electrophilic Selenium Catalysis with Electrophilic N
... that both NFSI and PhSeSePh decomposed gradually in the NMR experimental studies when PhSeSePh condition of PhSeBr as the catalyst, no imidated product was generated. Furthermore, it was found consumed completely. Thus, the authors speculated that the Se-Se bond did not break to form was mixed with ...
... that both NFSI and PhSeSePh decomposed gradually in the NMR experimental studies when PhSeSePh condition of PhSeBr as the catalyst, no imidated product was generated. Furthermore, it was found consumed completely. Thus, the authors speculated that the Se-Se bond did not break to form was mixed with ...
Chemistry 217 Problem Set 3 Recommended Problems from the Book
... of the carbons are attached to four groups, which should make them sp3-hybridized. However, the bond angles of the three-membered ring must be 60°, an angle more consistent with an sp2 hybridized carbon than an sp3, which has bond angles of 109.5°. 5. IR spectroscopy is commonly used by crime labs t ...
... of the carbons are attached to four groups, which should make them sp3-hybridized. However, the bond angles of the three-membered ring must be 60°, an angle more consistent with an sp2 hybridized carbon than an sp3, which has bond angles of 109.5°. 5. IR spectroscopy is commonly used by crime labs t ...
98 pts
... • (T) All E1 reactions involve formation of carbocations; • (T) More stable carbocations are generated faster; • (T) Carbocations are electrophiles; • (T) Carbocations are electron deficient; • (T) Free radicals are electron deficient; • (T) Alcohols are Brønsted bases; • (F) The rate-determining st ...
... • (T) All E1 reactions involve formation of carbocations; • (T) More stable carbocations are generated faster; • (T) Carbocations are electrophiles; • (T) Carbocations are electron deficient; • (T) Free radicals are electron deficient; • (T) Alcohols are Brønsted bases; • (F) The rate-determining st ...
7: Reactions of Haloalkanes, Alcohols, and Amines. Nucleophilic
... In fact, when R3 C-Br has fewer than two CH3 groups, it does not react at all by the S N1 mechanism (see Figure7.13). These changes in SN1 rates result from the effect of alkyl groups such as CH3 on the stability of R3 C+ that forms in the first step of the SN1 mechanism. Carbocation Stability. The ...
... In fact, when R3 C-Br has fewer than two CH3 groups, it does not react at all by the S N1 mechanism (see Figure7.13). These changes in SN1 rates result from the effect of alkyl groups such as CH3 on the stability of R3 C+ that forms in the first step of the SN1 mechanism. Carbocation Stability. The ...
Get PDF - Wiley Online Library
... outgrowth in primary cultured rat cortical neurons at concentrations as low as 10 nm. Given the important regulatory role of neurotrophins in the central nervous system, jiadifenolide represents a valuable small-molecule lead for the potential therapeutic treatment of neurodegenerative conditions su ...
... outgrowth in primary cultured rat cortical neurons at concentrations as low as 10 nm. Given the important regulatory role of neurotrophins in the central nervous system, jiadifenolide represents a valuable small-molecule lead for the potential therapeutic treatment of neurodegenerative conditions su ...
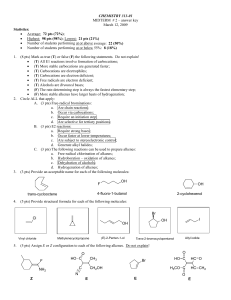
NAME Chem 204 Spring, 1990 Final Exam FINAL EXAM: Chaps. 1
... one of one reaction and one of two reactions) for converting a chiral alcohol into its inverted alkyl chloride, there is NO one reaction that will convert a chiral alcohol into its alkyl chloride with "retained" configuration. Thus, it is necessary to use several reaction steps, each of 100% inversi ...
... one of one reaction and one of two reactions) for converting a chiral alcohol into its inverted alkyl chloride, there is NO one reaction that will convert a chiral alcohol into its alkyl chloride with "retained" configuration. Thus, it is necessary to use several reaction steps, each of 100% inversi ...
PowerPoint 簡報
... Compounds containing aromatic rings are often used in dyes, such as these for sale in a market in Nepal ...
... Compounds containing aromatic rings are often used in dyes, such as these for sale in a market in Nepal ...
Stereoselective Construction of a β
... React. 1994, 46, 105-209. (c) Nakai, T.; Tomooka, K. Pure Appl. Chem. ...
... React. 1994, 46, 105-209. (c) Nakai, T.; Tomooka, K. Pure Appl. Chem. ...
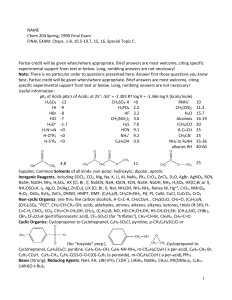
12_chemistry_impq_CH10_haloalkanes_and_haloarenes_02
... Q8. Why Grignard reagent should be prepared under an hydrous conditions.? Ans. Grignard reagent react with H2O to form alkanes , therefore they are prepared under anhydrous condition. Q9. Why is Sulphuric acid not used during the reaction of alcohols with KI ? ...
... Q8. Why Grignard reagent should be prepared under an hydrous conditions.? Ans. Grignard reagent react with H2O to form alkanes , therefore they are prepared under anhydrous condition. Q9. Why is Sulphuric acid not used during the reaction of alcohols with KI ? ...
Corrosion and Corrosion Enhancers in Amine Systems
... The remaining factors in the overall corrosion rate are those that prevent the corrosion products from forming a protective layer or those that cause the layer to be removed exposing more free metal. These are the other two factors in the overall corrosion rate expression. In the absence of H2S, “we ...
... The remaining factors in the overall corrosion rate are those that prevent the corrosion products from forming a protective layer or those that cause the layer to be removed exposing more free metal. These are the other two factors in the overall corrosion rate expression. In the absence of H2S, “we ...
Ester - Net Texts
... • using the alcohol in large excess (i.e., as a solvent) • using a dehydrating agent: Sulfuric acid not only catalyzes the reaction but sequesters water (a reaction product). Other drying agents like molecular sieves can also be used. • removal of water by physical means such as distillation as a lo ...
... • using the alcohol in large excess (i.e., as a solvent) • using a dehydrating agent: Sulfuric acid not only catalyzes the reaction but sequesters water (a reaction product). Other drying agents like molecular sieves can also be used. • removal of water by physical means such as distillation as a lo ...
aldehydes and ketones
... The reactivity of the carbonyl group towards the addition reaction depends upon the magnitude of the positive charge on the carbonyl carbon atom. Hence, any substituent that increases the positive charge on the carbonyl carbon must increase its reactivity towards addition reactions. The introduction ...
... The reactivity of the carbonyl group towards the addition reaction depends upon the magnitude of the positive charge on the carbonyl carbon atom. Hence, any substituent that increases the positive charge on the carbonyl carbon must increase its reactivity towards addition reactions. The introduction ...
Resolution of Diols via Catalytic Asymmetric Acetalization
... encouraged us to also explore the kinetic resolution of dialkylsubstituted diols. Gratifyingly, the reaction of methyl,cyclohexylsubstituted diol rac-2o proceeded with a selectivity factor of 23 (entry 9). With methyl,hexyl-substituted diol, the reaction still proceeded with a reasonable selectivity ...
... encouraged us to also explore the kinetic resolution of dialkylsubstituted diols. Gratifyingly, the reaction of methyl,cyclohexylsubstituted diol rac-2o proceeded with a selectivity factor of 23 (entry 9). With methyl,hexyl-substituted diol, the reaction still proceeded with a reasonable selectivity ...
SQA CfE Higher Chemistry Unit 2
... • an ester can be named given the names of the parent carboxylic acid and alcohol or from structural formulae; • structural formulae for esters can be drawn given the names of the parent alcohol and carboxylic acid or the names of esters; • esters have characteristic smells and are used as flavourin ...
... • an ester can be named given the names of the parent carboxylic acid and alcohol or from structural formulae; • structural formulae for esters can be drawn given the names of the parent alcohol and carboxylic acid or the names of esters; • esters have characteristic smells and are used as flavourin ...
Petasis reaction

The Petasis reaction (alternatively called the Petasis borono–Mannich (PBM) reaction) is the chemical reaction of an amine, aldehyde, and vinyl- or aryl-boronic acid to form substituted amines.Reported in 1993 by Nicos Petasis as a practical method towards the synthesis of a geometrically pure antifungal agent, naftifine, the Petasis reaction can be described as a variation of the Mannich reaction. Rather than generating an enolate to form the substituted amine product, in the Petasis reaction, the vinyl group of the organoboronic acid serves as the nucleophile. In comparison to other methods of generating allyl amines, the Petasis reaction tolerates a multifunctional scaffold, with a variety of amines and organoboronic acids as potential starting materials. Additionally, the reaction does not require anhydrous or inert conditions. As a mild, selective synthesis, the Petasis reaction is useful in generating α-amino acids, and is utilized in combinatorial chemistry and drug discovery.