
Limitations in Determining Enantiomeric Excess of Alcohols by 31P
... assuming that meso 1/meso 2 ratio follows the same value observed for the racemic mixture (Table 1). This strategy was necessary because the meso 2 signal, in this particular example, is overlapped by the monoalkyl phosphonated derivative (Table 1, Figs. 2d and 2e). This result was compatible with t ...
... assuming that meso 1/meso 2 ratio follows the same value observed for the racemic mixture (Table 1). This strategy was necessary because the meso 2 signal, in this particular example, is overlapped by the monoalkyl phosphonated derivative (Table 1, Figs. 2d and 2e). This result was compatible with t ...
Full-Text PDF
... Abstract: A new and highly efficient method mediated by tetrakis(acetonitrile)copper(I) triflate for activating both simple and highly hindered anhydrides in the acylation of alcohols and polyols is described. This new acylation method is mild and mostly proceeds at room temperature with low catalys ...
... Abstract: A new and highly efficient method mediated by tetrakis(acetonitrile)copper(I) triflate for activating both simple and highly hindered anhydrides in the acylation of alcohols and polyols is described. This new acylation method is mild and mostly proceeds at room temperature with low catalys ...
Changing counterion can switch the preference for selective 1,2
... Uncatalysed [4+2] and [3+2] cycloaddition processes typically feature inherent selectivity based on the electronic properties of the components involved. However, this inbuilt selectivity is rarely complete, and is often challenging to control. However, catalytic methods for divergent regioselective ...
... Uncatalysed [4+2] and [3+2] cycloaddition processes typically feature inherent selectivity based on the electronic properties of the components involved. However, this inbuilt selectivity is rarely complete, and is often challenging to control. However, catalytic methods for divergent regioselective ...
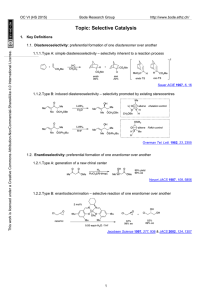
215-216 HH W12-notes
... Can be cleaved by heating with HI (more common) or HBr. Need a strong Brönsted or Lewis acid and a strong nucleophile. Usually, methyl ether C-O and benzyl ether C-O are those that can be cleaved. Modern methods for cleaving ethers include the use of BBr3 and AlCl3 + HSCH2CH3. O ...
... Can be cleaved by heating with HI (more common) or HBr. Need a strong Brönsted or Lewis acid and a strong nucleophile. Usually, methyl ether C-O and benzyl ether C-O are those that can be cleaved. Modern methods for cleaving ethers include the use of BBr3 and AlCl3 + HSCH2CH3. O ...
Alcohols - WordPress.com
... Alcohols are weak Brønsted bases Protonated by strong acids to yield oxonium ions, ...
... Alcohols are weak Brønsted bases Protonated by strong acids to yield oxonium ions, ...
Synthetic Applications of Zinc Borohydride
... others, asymmetric synthesis,22 peptide and pharmaceutical chemistry,23 and the synthesis of insecticidal compounds.24 Earlier preparative methods used reduction of esters of amino acids by sodium in ethanol.25 Subsequently, LiAlH426 and NaBH427 were used for the reduction of esters. Moreover, reduc ...
... others, asymmetric synthesis,22 peptide and pharmaceutical chemistry,23 and the synthesis of insecticidal compounds.24 Earlier preparative methods used reduction of esters of amino acids by sodium in ethanol.25 Subsequently, LiAlH426 and NaBH427 were used for the reduction of esters. Moreover, reduc ...
T_AllylCF3paperBM[5]
... still rare type of fluorinated species exhibited high electrophilicity and selectivity.3 The present work is a continuation of our investigations on electrophilic activation of alkenes4 and alkynes.5 Reactions of CF3-substituted allyl alcohols 1 promoted by Bronsted or Lewis acids were investigated ...
... still rare type of fluorinated species exhibited high electrophilicity and selectivity.3 The present work is a continuation of our investigations on electrophilic activation of alkenes4 and alkynes.5 Reactions of CF3-substituted allyl alcohols 1 promoted by Bronsted or Lewis acids were investigated ...
Chapter 19. Aldehydes and Ketones
... addition forming the conjugate acid of C=O Addition yields a hydroxy ether, called a hemiacetal (reversible); further reaction can occur Protonation of the OH and loss of water leads to an oxonium ion, R2C=OR+ to which a second alcohol adds to form the acetal ...
... addition forming the conjugate acid of C=O Addition yields a hydroxy ether, called a hemiacetal (reversible); further reaction can occur Protonation of the OH and loss of water leads to an oxonium ion, R2C=OR+ to which a second alcohol adds to form the acetal ...
Organic Chemistry II Introduction
... Reaction with hydrazine gives hydrazones – Reduction of hydrazone in base yields an alkane – Reduction of hydrazone in acid/Zn yields an alkane Alcohols add to yield acetals Phosphoranes add to aldehydes and ketones to give alkenes (the ...
... Reaction with hydrazine gives hydrazones – Reduction of hydrazone in base yields an alkane – Reduction of hydrazone in acid/Zn yields an alkane Alcohols add to yield acetals Phosphoranes add to aldehydes and ketones to give alkenes (the ...
07. Aldehydes and ketones
... In a strong acidic medium ketones give crotone condensation with formation of unsaturated ketones. ...
... In a strong acidic medium ketones give crotone condensation with formation of unsaturated ketones. ...
Chapter 1 Structure and Bonding
... 2) No Hydrogen Bonding is possible in R—O—R 3) Boiling Points are much lower than alcohols, more like haloalkanes 4) Water solubility much less than alcohols a) MeOMe and EtOEt have some water solubility b) Larger ethers are insoluble, very much like alkanes 5) Fairly unreactive, nonpolar solvents f ...
... 2) No Hydrogen Bonding is possible in R—O—R 3) Boiling Points are much lower than alcohols, more like haloalkanes 4) Water solubility much less than alcohols a) MeOMe and EtOEt have some water solubility b) Larger ethers are insoluble, very much like alkanes 5) Fairly unreactive, nonpolar solvents f ...
top organomet chem-2006-19-207 pauson
... ies support this mechanism while it explains the regio- and stereochemical results of numerous examples. Thus, Nakamura [53] and Milet and Gimbert [54] have performed high-level theoretical calculations on the cobaltacycle formation step, showing that the insertion of the olefin is the critical stere ...
... ies support this mechanism while it explains the regio- and stereochemical results of numerous examples. Thus, Nakamura [53] and Milet and Gimbert [54] have performed high-level theoretical calculations on the cobaltacycle formation step, showing that the insertion of the olefin is the critical stere ...
- Sacramento - California State University
... Vanadium has been used as a catalyst for polymerization1, oxidation of alcohols2, sulfides3, and more importantly, allylic alcohols. Epoxides are useful building blocks for natural product synthesis and medicinal chemistry because new functional groups can easily be introduced by nucleophilic additi ...
... Vanadium has been used as a catalyst for polymerization1, oxidation of alcohols2, sulfides3, and more importantly, allylic alcohols. Epoxides are useful building blocks for natural product synthesis and medicinal chemistry because new functional groups can easily be introduced by nucleophilic additi ...
10.3 PREPARATION OF ETHERS
... weaker base and a better leaving group. However, this intermediate is not isolated. Instead, it reacts immediately with the nucleophilic chloride ion that is generated during its formation. (The mechanism may be SN1 or SN2, depending on the structure of the compound.) The leaving group then decompos ...
... weaker base and a better leaving group. However, this intermediate is not isolated. Instead, it reacts immediately with the nucleophilic chloride ion that is generated during its formation. (The mechanism may be SN1 or SN2, depending on the structure of the compound.) The leaving group then decompos ...
The bite angle makes the catalyst
... of the allyl moiety. In the transition state, the hybridisation of this carbon atom changes from sp2 to sp3, which results in a bending of the propyl group towards the phosphine. This causes steric interference of the propyl group with the diphosphine ligand. The positive effect of large bite angles ...
... of the allyl moiety. In the transition state, the hybridisation of this carbon atom changes from sp2 to sp3, which results in a bending of the propyl group towards the phosphine. This causes steric interference of the propyl group with the diphosphine ligand. The positive effect of large bite angles ...
9851a doc..9851a chapter .. Page97
... Although TPAP is at its most active when absolutely fresh, and activity is lost almost immediately, we have shown that there is minimal further loss between one and six days after synthesis, if stored at low temperatures (Table 1). Further to this, UV analysis of TPAP showed little change in either ...
... Although TPAP is at its most active when absolutely fresh, and activity is lost almost immediately, we have shown that there is minimal further loss between one and six days after synthesis, if stored at low temperatures (Table 1). Further to this, UV analysis of TPAP showed little change in either ...
Organic synthesis and methodology related to the malaria drug artemisinin
... every year. Part 1 of this dissertation will focus on the history of Malaria and ways to combat this devastating disease. Artemisinin has emerged as the drug of choice for treatment of malaria due to its effectiveness against all strains of the malaria parasite. Access to artemisinin through isolati ...
... every year. Part 1 of this dissertation will focus on the history of Malaria and ways to combat this devastating disease. Artemisinin has emerged as the drug of choice for treatment of malaria due to its effectiveness against all strains of the malaria parasite. Access to artemisinin through isolati ...
Discodermolide

(+)-Discodermolide is a polyketide natural product found to stabilize microtubule. (+)-discodermolide was isolated by Gunasekera and his co-workers at the Harbor Branch Oceanographic Institute from the deep-sea sponge Discodermia dissoluta in 1990. (+)-Discodermolide was found to be a potent inhibitor of tumor cell growth in several MDR cancer cell lines. (+)-discodermolide also shows some unique characters, including a linear backbone structure, immunosuppressive properties both in vitro and in vivo, potent induction of an accelerated senescence phenotype, and synergistic antiproliferative activity in combination with paclitaxel. Discodermolide was recognized as one of the most potent natural promoters of tubulin assembly. A large number of efforts toward the total synthesis of (+)-discodermolide were directed by its interesting biological activities and extreme scarcity of natural sources (0.002% w/w from frozen marine sponge). The compound supply necessary for complete clinical trials cannot be met by harvesting, isolation, and purification. As of 2005, attempts at synthesis or semi-synthesis by fermentation have proven unsuccessful. As a result, all discodermolide used in preclinical studies and clinical trials has come from large-scale total synthesis.






![T_AllylCF3paperBM[5]](http://s1.studyres.com/store/data/003584459_1-3decab572f7fca68901a941affab18ea-300x300.png)















