File - Rasapalli Research Group
... 3. Know reactions and reagents for preparation of alcohols including review reactions. 4. Know reactions of alcohols and mechanisms. ...
... 3. Know reactions and reagents for preparation of alcohols including review reactions. 4. Know reactions of alcohols and mechanisms. ...
IOSR Journal of Applied Chemistry (IOSR-JAC) e-ISSN: 2278-5736.
... physical and chemical properties of transition metal complexes are greatly influenced by their structures and the coordination geometries by the ligand design. Transition metal complexes and Schiff bases derived from the reaction of aromatic aldehyde and aliphatic or aromatic amines represents an im ...
... physical and chemical properties of transition metal complexes are greatly influenced by their structures and the coordination geometries by the ligand design. Transition metal complexes and Schiff bases derived from the reaction of aromatic aldehyde and aliphatic or aromatic amines represents an im ...
Recent developments in the applications of palladium complexes
... 2.2. Pd-catalyzed reactions in alternative solvents i) Hydrophilic ligands and aqueous Pd-catalyzed reactions. The use of water as solvent has attracted great interest for economical and ecological reasons and so, water-soluble homogenous catalysts are highly desirable. This strategy requires that t ...
... 2.2. Pd-catalyzed reactions in alternative solvents i) Hydrophilic ligands and aqueous Pd-catalyzed reactions. The use of water as solvent has attracted great interest for economical and ecological reasons and so, water-soluble homogenous catalysts are highly desirable. This strategy requires that t ...
Reaction of Organometallic Reagents with Aldehydes and Ketones.
... • The difference between the two reactions is what then happens to the intermediate. • Aldehydes and ketones cannot undergo substitution because they do not have a good leaving group bonded to the newly formed sp3 hybridized carbon. ...
... • The difference between the two reactions is what then happens to the intermediate. • Aldehydes and ketones cannot undergo substitution because they do not have a good leaving group bonded to the newly formed sp3 hybridized carbon. ...
- Opus: Online Publications Store
... bond in a molecule it is necessary to activate this bond (making it more reactive) as the first step, followed by its subsequent functionalization [1a]. The activation process can be achieved effectively by using transition metal catalysts and others [1c,11]. It is well−known that complexes based on ...
... bond in a molecule it is necessary to activate this bond (making it more reactive) as the first step, followed by its subsequent functionalization [1a]. The activation process can be achieved effectively by using transition metal catalysts and others [1c,11]. It is well−known that complexes based on ...
CHAPTER V Fischer-Tropsch Syncrude
... hydrocarbons (>C20), do not have a single α-value, but two. The first α-value (α1) describes the carbon number distribution from C3 to C12, while the second α-value (α2) describes the distribution of the C20 and heavier fraction.(4) This seems to be a mathematical convenience, since the α-value seem ...
... hydrocarbons (>C20), do not have a single α-value, but two. The first α-value (α1) describes the carbon number distribution from C3 to C12, while the second α-value (α2) describes the distribution of the C20 and heavier fraction.(4) This seems to be a mathematical convenience, since the α-value seem ...
WADE7Lecture10a
... The longest chain contains six carbon atoms, but it does not contain the carbon bonded to the hydroxyl group. The longest chain containing the carbon bonded to the —OH group is the one outlined by the green box, containing five carbon atoms. This chain is numbered from right to left in order to give ...
... The longest chain contains six carbon atoms, but it does not contain the carbon bonded to the hydroxyl group. The longest chain containing the carbon bonded to the —OH group is the one outlined by the green box, containing five carbon atoms. This chain is numbered from right to left in order to give ...
Crocus sativus
... Behnia (1992) stated that the number of saffron corms required per unit of land depends on the planting method, size of corms and also the habit of farmers, and varies between 1.5 to 10 tons per hectare. Alavi-shahri et al. (1995) reported that by increasing plant density stigma dry matter yield was ...
... Behnia (1992) stated that the number of saffron corms required per unit of land depends on the planting method, size of corms and also the habit of farmers, and varies between 1.5 to 10 tons per hectare. Alavi-shahri et al. (1995) reported that by increasing plant density stigma dry matter yield was ...
paper - General Guide To Personal and Societies Web Space at
... starting point was homoallylglycine (Hag) since its in ViVo incorporation by methionine auxotrophic Escherichia coli is known.4 Hoveyda-Grubbs second generation catalyst 17 was selected since it is phosphine free and therefore more likely to be compatible with protein disulfides than other conventio ...
... starting point was homoallylglycine (Hag) since its in ViVo incorporation by methionine auxotrophic Escherichia coli is known.4 Hoveyda-Grubbs second generation catalyst 17 was selected since it is phosphine free and therefore more likely to be compatible with protein disulfides than other conventio ...
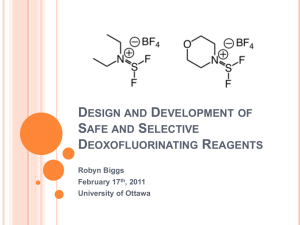
Design and Development of Safe and Selective Deoxofluorinating
... Highly toxic and corrosive Typically requires high temperatures (>100°C) Low selectivity ...
... Highly toxic and corrosive Typically requires high temperatures (>100°C) Low selectivity ...
Document
... E.Q.: What mathematical relationships can be determined from a balanced chemical equation? ...
... E.Q.: What mathematical relationships can be determined from a balanced chemical equation? ...
Organic Chemistry – Summary of Reactions and Conditions
... contain copper (II) complexes. These complexes enable Cu (II) to remain in solution in the presence of alkali. Ketones are resistant to oxidation and do not react with Benedict's solution. Tollen's reagent (ammoniacal silver nitrate) (not a preparative method) Prepared by adding excess ammonia solut ...
... contain copper (II) complexes. These complexes enable Cu (II) to remain in solution in the presence of alkali. Ketones are resistant to oxidation and do not react with Benedict's solution. Tollen's reagent (ammoniacal silver nitrate) (not a preparative method) Prepared by adding excess ammonia solut ...
Mead Chemistry Lap 11: Stoichiometry Chapter 12 12.1 Balanced
... • Consider: N2 +3H2 2 NH3 • Number of atoms ▫ Can count the number of atoms on both sides of an equation ▫ 2 atoms of N, 6 atoms of H • Number of molecules ▫ Use coefficients to find # of molecules ▫ 1 molecule N2 reacts with 3 molecules H2 to form 2 molecules of NH3 • Number of moles ▫ Use coeffic ...
... • Consider: N2 +3H2 2 NH3 • Number of atoms ▫ Can count the number of atoms on both sides of an equation ▫ 2 atoms of N, 6 atoms of H • Number of molecules ▫ Use coefficients to find # of molecules ▫ 1 molecule N2 reacts with 3 molecules H2 to form 2 molecules of NH3 • Number of moles ▫ Use coeffic ...
Recent advances in homogeneous nickel catalysis
... have been disclosed. Benzylic cross-coupling Another mode of bond activation that has come to prominence, at least in part thanks to nickel catalysis, is the activation of benzylic C–O bonds37. Benzylic ethers, esters, carbonates, carbamates and, in some instances, even free alcohols (via the corre ...
... have been disclosed. Benzylic cross-coupling Another mode of bond activation that has come to prominence, at least in part thanks to nickel catalysis, is the activation of benzylic C–O bonds37. Benzylic ethers, esters, carbonates, carbamates and, in some instances, even free alcohols (via the corre ...
Aldehydes and Ketones
... If the CHO group is bonded to a ring, name the ring and add the suffix –carbaldehyde. Number the chain or ring to put the CHO group at C1, but omit this number from the name. Apply all the other usual rules of nomenclature. ...
... If the CHO group is bonded to a ring, name the ring and add the suffix –carbaldehyde. Number the chain or ring to put the CHO group at C1, but omit this number from the name. Apply all the other usual rules of nomenclature. ...
IOSR Journal of Applied Chemistry (IOSR-JAC)
... transformation [1]. Diverse reagents and reaction conditions have been developed for this purpose. Conversion of aromatic amines to corresponding acetamides is also well documented [2]. Reduction of nitro group and acetylation of the resulting amine are often carried out in succession to attenuate t ...
... transformation [1]. Diverse reagents and reaction conditions have been developed for this purpose. Conversion of aromatic amines to corresponding acetamides is also well documented [2]. Reduction of nitro group and acetylation of the resulting amine are often carried out in succession to attenuate t ...
Nomenclature
... Best for making symmetrical ethers formed from unbranched primary alcohols. Industrial method, not good lab synthesis. If temp. is too high, alkene forms, elimination predominates with 2° and 3° alcohols. Diols can cyclize to form 5- or 6-membered rings. ...
... Best for making symmetrical ethers formed from unbranched primary alcohols. Industrial method, not good lab synthesis. If temp. is too high, alkene forms, elimination predominates with 2° and 3° alcohols. Diols can cyclize to form 5- or 6-membered rings. ...
10. Alkyl Halides
... If the concentration of alkyl halide is doubled, halfed or quadrupled the reaction rate will double, half or quadruple. If, on the other hand, the concentration of nucleophile is changed the reaction rate will be unaffected If the rate of this reaction does not depend upon the concentration of t ...
... If the concentration of alkyl halide is doubled, halfed or quadrupled the reaction rate will double, half or quadruple. If, on the other hand, the concentration of nucleophile is changed the reaction rate will be unaffected If the rate of this reaction does not depend upon the concentration of t ...
3.2 Organic Synthesis (Reaction Pathways)
... Also covered in Higher Chemistry, the production of an alkylhalide can be a significan step in Synthesis as it opens up the route to a variety of other molecules - ethers, alcohols, amines, acids etc. Alkynes will have a similar addition reaction but will be able to react with 2 moles of halogen. ...
... Also covered in Higher Chemistry, the production of an alkylhalide can be a significan step in Synthesis as it opens up the route to a variety of other molecules - ethers, alcohols, amines, acids etc. Alkynes will have a similar addition reaction but will be able to react with 2 moles of halogen. ...
Discodermolide

(+)-Discodermolide is a polyketide natural product found to stabilize microtubule. (+)-discodermolide was isolated by Gunasekera and his co-workers at the Harbor Branch Oceanographic Institute from the deep-sea sponge Discodermia dissoluta in 1990. (+)-Discodermolide was found to be a potent inhibitor of tumor cell growth in several MDR cancer cell lines. (+)-discodermolide also shows some unique characters, including a linear backbone structure, immunosuppressive properties both in vitro and in vivo, potent induction of an accelerated senescence phenotype, and synergistic antiproliferative activity in combination with paclitaxel. Discodermolide was recognized as one of the most potent natural promoters of tubulin assembly. A large number of efforts toward the total synthesis of (+)-discodermolide were directed by its interesting biological activities and extreme scarcity of natural sources (0.002% w/w from frozen marine sponge). The compound supply necessary for complete clinical trials cannot be met by harvesting, isolation, and purification. As of 2005, attempts at synthesis or semi-synthesis by fermentation have proven unsuccessful. As a result, all discodermolide used in preclinical studies and clinical trials has come from large-scale total synthesis.