Contents - Personal WWW Pages
... 1.2 Homogeneous vs Heterogeneous Catalysis. It was Sabatier who, in 1927, published the first classification of catalysts and used the terms homogeneous and heterogeneous. A heterogeneous catalyst exists in a separate phase to the reaction medium (most commonly as a solid in either a liquid or gaseo ...
... 1.2 Homogeneous vs Heterogeneous Catalysis. It was Sabatier who, in 1927, published the first classification of catalysts and used the terms homogeneous and heterogeneous. A heterogeneous catalyst exists in a separate phase to the reaction medium (most commonly as a solid in either a liquid or gaseo ...
Chapter 19. Aldehydes and Ketones: Nucleophilic Addition Reactions
... a dipolar intermediate called a betaine The intermediate spontaneously decomposes through a four-membered ring to yield alkene and triphenylphosphine oxide, (Ph)3P=O Formation of the ylide is shown below ...
... a dipolar intermediate called a betaine The intermediate spontaneously decomposes through a four-membered ring to yield alkene and triphenylphosphine oxide, (Ph)3P=O Formation of the ylide is shown below ...
Exam 2
... 7. (16 pts -8 pts each) For the reactions below, state if the mechanism is Sn1, Sn2, E2 or E 1 and draw the product( s) produced by this mechanism. Be sure to show the pertinent stereochemistry for the product(s). ...
... 7. (16 pts -8 pts each) For the reactions below, state if the mechanism is Sn1, Sn2, E2 or E 1 and draw the product( s) produced by this mechanism. Be sure to show the pertinent stereochemistry for the product(s). ...
Chapter 13 Silicon reagents
... • Silicon does not form very stable multiple bonds, as the large 3p orbital on silicon does not overlap well with the 2p orbital on carbon, oxygen or nitrogen. • Carbon is more electronegative than silicon •Silicon is a very versatile element, and you will find silicon reagents in 2 major roles; •As ...
... • Silicon does not form very stable multiple bonds, as the large 3p orbital on silicon does not overlap well with the 2p orbital on carbon, oxygen or nitrogen. • Carbon is more electronegative than silicon •Silicon is a very versatile element, and you will find silicon reagents in 2 major roles; •As ...
Solved Examples
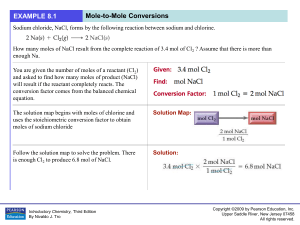
... In photosynthesis, plants convert carbon dioxide and water into glucose (C 6 H12O6) according to the following reaction. How many grams of glucose can be synthesized from 58.5 g of CO2 ? Assume that there is more than enough water present to react with all of the CO2 . Set up the problem in the norm ...
... In photosynthesis, plants convert carbon dioxide and water into glucose (C 6 H12O6) according to the following reaction. How many grams of glucose can be synthesized from 58.5 g of CO2 ? Assume that there is more than enough water present to react with all of the CO2 . Set up the problem in the norm ...
04_01_03.html
... Overview of Chapter This chapter introduces chemical reactions and their mechanisms by focusing on two reactions that yield alkyl halides. (1) alcohol + hydrogen halide ROH + HX RX + H2O (2) alkane + halogen RH + X2 RX + HX Both are substitution reactions ...
... Overview of Chapter This chapter introduces chemical reactions and their mechanisms by focusing on two reactions that yield alkyl halides. (1) alcohol + hydrogen halide ROH + HX RX + H2O (2) alkane + halogen RH + X2 RX + HX Both are substitution reactions ...
Ch. 09 Alcohols, Ethers, Epoxides
... Reaction of Ethers with Strong Acid • In order for ethers to undergo substitution or elimination reactions, their poor leaving group must first be converted into a good leaving group by reaction with strong acids such as HBr and HI. • HBr and HI are strong acids that are also sources of good nucleo ...
... Reaction of Ethers with Strong Acid • In order for ethers to undergo substitution or elimination reactions, their poor leaving group must first be converted into a good leaving group by reaction with strong acids such as HBr and HI. • HBr and HI are strong acids that are also sources of good nucleo ...
INTRODUCING ALDEHYDES AND KETONES
... charged part of a molecule (for example, the lone pair on a nitrogen atom in ammonia, NH3). During the reaction, the carbon-oxygen double bond gets broken. The net effect of all this is that the carbonyl group undergoes addition reactions, often followed by the loss of a water molecule. This gives a ...
... charged part of a molecule (for example, the lone pair on a nitrogen atom in ammonia, NH3). During the reaction, the carbon-oxygen double bond gets broken. The net effect of all this is that the carbonyl group undergoes addition reactions, often followed by the loss of a water molecule. This gives a ...
Mechanism of the oxymercuration of substituted cyclohexenes
... tion of equatorial alcohols on reduction. The total amount of equatorial alcohol approached 79 % after 10 days at 25" (see Table I). During this period of time a precipitate slowly formed. Due to the presence of the insoluble material it was not possible to determine the stereochemical relationship ...
... tion of equatorial alcohols on reduction. The total amount of equatorial alcohol approached 79 % after 10 days at 25" (see Table I). During this period of time a precipitate slowly formed. Due to the presence of the insoluble material it was not possible to determine the stereochemical relationship ...
Acetal Formation
... The acetal is a functional group in which a carbon atom is bonded to two –OR groups Acetal formation is a condensation reaction between two hydroxyl groups and a ketone or aldehyde in which water is lost. Vocabulary Acetal Hemiacetal Diol Students should be able to: Identify the acetal a ...
... The acetal is a functional group in which a carbon atom is bonded to two –OR groups Acetal formation is a condensation reaction between two hydroxyl groups and a ketone or aldehyde in which water is lost. Vocabulary Acetal Hemiacetal Diol Students should be able to: Identify the acetal a ...
Preparation of Alkyl Halides
... Victor Grignard discovered that a dry alkyl halide will react with dry magnesium metal in a dry ether solvent to produce an organometallic compound with that behaves as if it has the structure R-Mg-X It is now called an alkylmagnesium halide or Grignard reagent: ...
... Victor Grignard discovered that a dry alkyl halide will react with dry magnesium metal in a dry ether solvent to produce an organometallic compound with that behaves as if it has the structure R-Mg-X It is now called an alkylmagnesium halide or Grignard reagent: ...
Full-Text PDF
... regarding the introduction of a formyl group into aromatic rings [3,6]; however, the organic chemist continues to face the challenge of formylation through C-C bond formation [23], which is the most convenient approach for the case of aromatic aldehydes. One of the most widely used procedures is the ...
... regarding the introduction of a formyl group into aromatic rings [3,6]; however, the organic chemist continues to face the challenge of formylation through C-C bond formation [23], which is the most convenient approach for the case of aromatic aldehydes. One of the most widely used procedures is the ...
Chapter 21 aldehydes and ketones
... • The sp2 hybridized C–H proton of an aldehyde is highly deshielded and absorbs far downfield at 9–10 ppm. • Splitting occurs with protons on the carbon, but the coupling constant is often very small (J = 1–3 Hz). • Protons on the carbon to the carbonyl group absorb at ...
... • The sp2 hybridized C–H proton of an aldehyde is highly deshielded and absorbs far downfield at 9–10 ppm. • Splitting occurs with protons on the carbon, but the coupling constant is often very small (J = 1–3 Hz). • Protons on the carbon to the carbonyl group absorb at ...
ENGLISH VERSION Exam Organic Chemistry 2
... A benzophenone structure was used as starting material in the synthesis of Meclizine mentioned above. Design a synthetic route to this substance starting from benzene and suitable reagents. The synthesis may proceed in many steps, but indicate possible weaknesses and byproducts in each step. (10p) O ...
... A benzophenone structure was used as starting material in the synthesis of Meclizine mentioned above. Design a synthetic route to this substance starting from benzene and suitable reagents. The synthesis may proceed in many steps, but indicate possible weaknesses and byproducts in each step. (10p) O ...
Synthesis of enantiopure alcohols
... esterifications of a range of secondary alcohols (1-4) catalyzed by immobilized lipase B from Candida antarctica (Novozym 435) and that addition of enantiopure (R)alcohols, (R)-1, (R)-2, (R)-5, (R)-6 and (R)-7, induced increase in the E-value of the esterification of 3-chloro-1-phenoxy-2-propanol (4 ...
... esterifications of a range of secondary alcohols (1-4) catalyzed by immobilized lipase B from Candida antarctica (Novozym 435) and that addition of enantiopure (R)alcohols, (R)-1, (R)-2, (R)-5, (R)-6 and (R)-7, induced increase in the E-value of the esterification of 3-chloro-1-phenoxy-2-propanol (4 ...
Document
... letting 12.00 moles of KClO3 react? 2. How many moles of KClO3 are needed to produce 5.45 moles of KCl? 3. If 10.4 moles of KCl were produced, how many moles of O2 were also produced? 4. How many moles of KCl can be produced by letting 7.5 moles of KClO3 decompose? ...
... letting 12.00 moles of KClO3 react? 2. How many moles of KClO3 are needed to produce 5.45 moles of KCl? 3. If 10.4 moles of KCl were produced, how many moles of O2 were also produced? 4. How many moles of KCl can be produced by letting 7.5 moles of KClO3 decompose? ...
Practice Problem - HCC Southeast Commons
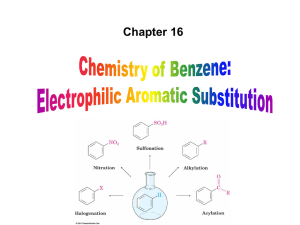
... resonance effects that reinforce each other – The ortho and para intermediates are destabilized – The positive charge of the carbocation intermediate in ortho and para attack is directly on the carbon that bears the deactivating group and resonance ...
... resonance effects that reinforce each other – The ortho and para intermediates are destabilized – The positive charge of the carbocation intermediate in ortho and para attack is directly on the carbon that bears the deactivating group and resonance ...
Stoichiometry - HCC Learning Web
... Step 3: Calculate the moles of "desired" substance from your answer in Step 2 using the coefficients from the balanced chemical equation. If more than one reactant was given originally, you can calculate the moles of product twice, based on the moles of each reactant. The reactant that gives the sma ...
... Step 3: Calculate the moles of "desired" substance from your answer in Step 2 using the coefficients from the balanced chemical equation. If more than one reactant was given originally, you can calculate the moles of product twice, based on the moles of each reactant. The reactant that gives the sma ...
Disproportionation of Monolithium Acetylide into
... most widely used ethynylation and alkynylation reaction.1 Monolithium acetylide (1) disproportionates readily above -25 °C into the more stable dilithium carbide (2) and acetylene.2 Liquid ammonia which is utilized in processes claimed to have industrial economics1,3 serves not only as a solvent but ...
... most widely used ethynylation and alkynylation reaction.1 Monolithium acetylide (1) disproportionates readily above -25 °C into the more stable dilithium carbide (2) and acetylene.2 Liquid ammonia which is utilized in processes claimed to have industrial economics1,3 serves not only as a solvent but ...
Discodermolide

(+)-Discodermolide is a polyketide natural product found to stabilize microtubule. (+)-discodermolide was isolated by Gunasekera and his co-workers at the Harbor Branch Oceanographic Institute from the deep-sea sponge Discodermia dissoluta in 1990. (+)-Discodermolide was found to be a potent inhibitor of tumor cell growth in several MDR cancer cell lines. (+)-discodermolide also shows some unique characters, including a linear backbone structure, immunosuppressive properties both in vitro and in vivo, potent induction of an accelerated senescence phenotype, and synergistic antiproliferative activity in combination with paclitaxel. Discodermolide was recognized as one of the most potent natural promoters of tubulin assembly. A large number of efforts toward the total synthesis of (+)-discodermolide were directed by its interesting biological activities and extreme scarcity of natural sources (0.002% w/w from frozen marine sponge). The compound supply necessary for complete clinical trials cannot be met by harvesting, isolation, and purification. As of 2005, attempts at synthesis or semi-synthesis by fermentation have proven unsuccessful. As a result, all discodermolide used in preclinical studies and clinical trials has come from large-scale total synthesis.