Chloroperbenzoic_aci..
... of (Z)-cyclooct-2-en-1-ol is anti selective; with TBHP/V it is cis selective.24b Similar differences have been noticed in some acyclic systems.27c Since the directing effect of the hydroxyl group is larger in the TBHP/V system it is a better reagent for hydroxyl-directed regioselective epoxidations ...
... of (Z)-cyclooct-2-en-1-ol is anti selective; with TBHP/V it is cis selective.24b Similar differences have been noticed in some acyclic systems.27c Since the directing effect of the hydroxyl group is larger in the TBHP/V system it is a better reagent for hydroxyl-directed regioselective epoxidations ...
Reactions involving HCl and their Evaporation
... can be difficult to remove, and/or the sample elutes as the TFA salt. In such cases a re-salt formation can be achieved by passing the collected sample through a Solid Phase Extraction (SPE) column to bind the sample to the column. By washing with an excess of base in solution the TFA salt is neutra ...
... can be difficult to remove, and/or the sample elutes as the TFA salt. In such cases a re-salt formation can be achieved by passing the collected sample through a Solid Phase Extraction (SPE) column to bind the sample to the column. By washing with an excess of base in solution the TFA salt is neutra ...
Abstract Isomorphous substitution of Al or Si in zeolites/molecular
... molecular sieves are a new class of materials which can also catalyze various oxidation reactions selectively. They have interesting catalytic properties in ammoxidation of xylenes and propane, oxidative dehydrogenation of propane, oxyfunctionalization of alkanes and hydroxylation of aromatics. This ...
... molecular sieves are a new class of materials which can also catalyze various oxidation reactions selectively. They have interesting catalytic properties in ammoxidation of xylenes and propane, oxidative dehydrogenation of propane, oxyfunctionalization of alkanes and hydroxylation of aromatics. This ...
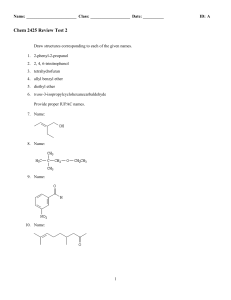
Chem 2425-Test 2 Review
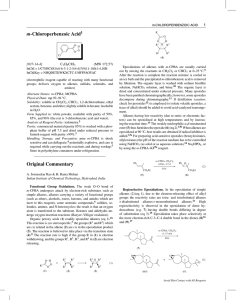
... Epoxides are synthesized industrially in one step by silver oxide air oxidation of ethylene and on a laboratory scale in one step by treating an alkene with m-chloroperoxybenzoic acid. An alternative two step process converts alkenes to halohydrins, which are converted by treatment with base to epox ...
... Epoxides are synthesized industrially in one step by silver oxide air oxidation of ethylene and on a laboratory scale in one step by treating an alkene with m-chloroperoxybenzoic acid. An alternative two step process converts alkenes to halohydrins, which are converted by treatment with base to epox ...
Chem 314 Preorganic Evaluation
... also each hydrogen of a C-beta CH 2 will often be different, producing E or Z stereoisomer alkenes depending on the anti conformations present, also a chiral 3o RX Cβ-H may have R ...
... also each hydrogen of a C-beta CH 2 will often be different, producing E or Z stereoisomer alkenes depending on the anti conformations present, also a chiral 3o RX Cβ-H may have R ...
Reactions You Should Know When You Begin Organic II
... Determine the number of constitutional isomers produced when a halogen is reacted with a higher alkane (more complex than simple alkane). ...
... Determine the number of constitutional isomers produced when a halogen is reacted with a higher alkane (more complex than simple alkane). ...
Chapter 23 - Simpson County Schools
... Extremely important to carbohydrate chemistry due to nearly all carbohydrates are found as ringed molecules because they form intramolecular hemiacetals or hemiketals. The acetal/ketal reaction provides the means by which carbohydrates link together to form the wellknown carbohydrate polymers such a ...
... Extremely important to carbohydrate chemistry due to nearly all carbohydrates are found as ringed molecules because they form intramolecular hemiacetals or hemiketals. The acetal/ketal reaction provides the means by which carbohydrates link together to form the wellknown carbohydrate polymers such a ...
Alkyl halide
... Allylic bromination of an unsymmetrical alkene often leads to a mixture of products • Products are not usually formed in equal amounts because the intermediate allylic radical is not symmetrical and reaction at the two ends is not equally likely ...
... Allylic bromination of an unsymmetrical alkene often leads to a mixture of products • Products are not usually formed in equal amounts because the intermediate allylic radical is not symmetrical and reaction at the two ends is not equally likely ...
Chapter 21: Amines. Organic derivatives of ammonia, NH3. Nitrogen
... Table 21.1 (p. 964): pKa values of ammonium ions Alkyl ammonium ions, R3NH+ X-, have pKa values in the range of 10-11 (ammonium ion, H4N+ X-, has a pKa ~ 9.3) The ammonium ions of aryl amines and heterocyclic aromatic amines are considerably more acidic than alkyl amines (pKa < 5). The nitrogen lone ...
... Table 21.1 (p. 964): pKa values of ammonium ions Alkyl ammonium ions, R3NH+ X-, have pKa values in the range of 10-11 (ammonium ion, H4N+ X-, has a pKa ~ 9.3) The ammonium ions of aryl amines and heterocyclic aromatic amines are considerably more acidic than alkyl amines (pKa < 5). The nitrogen lone ...
Organic Chemistry Introduction
... • Stabilize a high energy intermediate you stabilize the transition state leading to it ...
... • Stabilize a high energy intermediate you stabilize the transition state leading to it ...
Aldehydes, Ketones and Carboxylic acids
... Sterically, the presence of two bulky (large) groups in ketones will hinder the attack of nucleophile to carbonyl carbon in ketone. Aldehydes have only one bulky group around the carbonyl carbon and it is easier for the nucleophile to attack the carbonyl carbon as compared to ketones. Electronically ...
... Sterically, the presence of two bulky (large) groups in ketones will hinder the attack of nucleophile to carbonyl carbon in ketone. Aldehydes have only one bulky group around the carbonyl carbon and it is easier for the nucleophile to attack the carbonyl carbon as compared to ketones. Electronically ...
Organic compounds containing Nitrogen
... Ans. (i) Hoffmann bromamide degradation reaction: This is a method used for converting primary amide into primary amine. An aide is treated with bromine in an aqueous or ethanolic solution of sodium hydroxide. The amine so formed contains one carbon less than that present in the amide. RCONH 2 + Br2 ...
... Ans. (i) Hoffmann bromamide degradation reaction: This is a method used for converting primary amide into primary amine. An aide is treated with bromine in an aqueous or ethanolic solution of sodium hydroxide. The amine so formed contains one carbon less than that present in the amide. RCONH 2 + Br2 ...
HIGHLY SELECTIVE RHODIUM–CATALYZED C–H BORYLATIONS IN
... toss the ball around or stomp the boots and giddy up to some good ‘ol country tunes. Deepest thanks goes out to my family who were always standing beside me along the way. I would not have been able to make it this far without your reassurance and inspiration. It is not lost how instrumental you wer ...
... toss the ball around or stomp the boots and giddy up to some good ‘ol country tunes. Deepest thanks goes out to my family who were always standing beside me along the way. I would not have been able to make it this far without your reassurance and inspiration. It is not lost how instrumental you wer ...
New Stereoselective Approaches to Highly Substituted
... Barlett and Myerson6 first recorded the use of 5-endo-trig cyclisations in the synthesis of tetrahydrofurans in 1978. They conducted an iodolactonisation on methyl ester 5, which furnished a 2:1 mixture of the desired lactone 7 and an iodotetrahydrofuran 6 , via a competing iodoetherification reacti ...
... Barlett and Myerson6 first recorded the use of 5-endo-trig cyclisations in the synthesis of tetrahydrofurans in 1978. They conducted an iodolactonisation on methyl ester 5, which furnished a 2:1 mixture of the desired lactone 7 and an iodotetrahydrofuran 6 , via a competing iodoetherification reacti ...
PPT file
... Because electrophilic addition to a carbonyl group is reactantfavoured, whenever we see a gem-diol (two –OH groups on one carbon) or a carbon atom with a –OH group and a halogen attached, we expect it to collapse to a carbonyl group: H O C ...
... Because electrophilic addition to a carbonyl group is reactantfavoured, whenever we see a gem-diol (two –OH groups on one carbon) or a carbon atom with a –OH group and a halogen attached, we expect it to collapse to a carbonyl group: H O C ...
An Oxidation-Reduction Scheme: Borneol, Camphor, Isoborneol1
... Assemble the Apparatus. To a 10-mL round-bottom flask add 0.360 g of racemic borneol , 1.0 mL of acetone, and 0.30 mL of glacial acetic acid. After adding a magnetic stir bar to the flask, attach an air condenser and place the round-bottom flask in a water bath at about 45 °C. It is important that t ...
... Assemble the Apparatus. To a 10-mL round-bottom flask add 0.360 g of racemic borneol , 1.0 mL of acetone, and 0.30 mL of glacial acetic acid. After adding a magnetic stir bar to the flask, attach an air condenser and place the round-bottom flask in a water bath at about 45 °C. It is important that t ...
PPT File
... 10.5 Reaction of Amines Amines are less reactive than alcohols. • This can be evaluated by inspection of the pKa values of the leaving group. ...
... 10.5 Reaction of Amines Amines are less reactive than alcohols. • This can be evaluated by inspection of the pKa values of the leaving group. ...
- University at Albany
... Also, alkyl halide reactivity decreases from methyl to 10 to 20 to 30. In fact, 30 alkyl halides do not react by SN2. Leaving group: The substrate should have a good leaving group. A good leaving group should be electron withdrawing, relatively stable, and polarizable. They are weak bases. Examples ...
... Also, alkyl halide reactivity decreases from methyl to 10 to 20 to 30. In fact, 30 alkyl halides do not react by SN2. Leaving group: The substrate should have a good leaving group. A good leaving group should be electron withdrawing, relatively stable, and polarizable. They are weak bases. Examples ...
Reduction Reactions
... 3.1.3 Evans-Tishchenko Reduction D. A. Evans, A. H. Hoveyda, J. Am. Chem. Soc., 1990, 112, 6447-6449. ...
... 3.1.3 Evans-Tishchenko Reduction D. A. Evans, A. H. Hoveyda, J. Am. Chem. Soc., 1990, 112, 6447-6449. ...
Lectures 4-6
... Collins Oxidation (CrO3 • 2pyridine) TL 1969, 336 - CrO3 (anhydrous) + pyridine (anhydrous) ...
... Collins Oxidation (CrO3 • 2pyridine) TL 1969, 336 - CrO3 (anhydrous) + pyridine (anhydrous) ...
Chapter 8 I. Nucleophilic Substitution
... Physicist Roland Wester and his team in Matthias Weidemüller's group at the University of Freiburg, in Germany, in collaboration with William L. Hase's group at Texas Tech University, provide direct evidence for this mechanism in the gas phase. However, they also detected an additional, unexpected m ...
... Physicist Roland Wester and his team in Matthias Weidemüller's group at the University of Freiburg, in Germany, in collaboration with William L. Hase's group at Texas Tech University, provide direct evidence for this mechanism in the gas phase. However, they also detected an additional, unexpected m ...
Full Article - PDF - Brandeis University
... Department of Chemistry, Brandeis UniVersity Waltham, Massachusetts 02454-9110 ReceiVed August 9, 2001 Acyl-transfer reactions use cheap reagents to transform readily available starting materials into useful and easily purified products. These characteristics, in combination with high enantioselecti ...
... Department of Chemistry, Brandeis UniVersity Waltham, Massachusetts 02454-9110 ReceiVed August 9, 2001 Acyl-transfer reactions use cheap reagents to transform readily available starting materials into useful and easily purified products. These characteristics, in combination with high enantioselecti ...
Discodermolide

(+)-Discodermolide is a polyketide natural product found to stabilize microtubule. (+)-discodermolide was isolated by Gunasekera and his co-workers at the Harbor Branch Oceanographic Institute from the deep-sea sponge Discodermia dissoluta in 1990. (+)-Discodermolide was found to be a potent inhibitor of tumor cell growth in several MDR cancer cell lines. (+)-discodermolide also shows some unique characters, including a linear backbone structure, immunosuppressive properties both in vitro and in vivo, potent induction of an accelerated senescence phenotype, and synergistic antiproliferative activity in combination with paclitaxel. Discodermolide was recognized as one of the most potent natural promoters of tubulin assembly. A large number of efforts toward the total synthesis of (+)-discodermolide were directed by its interesting biological activities and extreme scarcity of natural sources (0.002% w/w from frozen marine sponge). The compound supply necessary for complete clinical trials cannot be met by harvesting, isolation, and purification. As of 2005, attempts at synthesis or semi-synthesis by fermentation have proven unsuccessful. As a result, all discodermolide used in preclinical studies and clinical trials has come from large-scale total synthesis.