Synopsis
... Toward this end, debromination of 57 was effected by treatment with ntributyltin hydride in refluxing benzene in the presence of cat. amounts of AIBN to furnish acetonide 58. Deprotection of the acetonide using cat. CSA in methanol proceeded cleanly to yield alcohol 59 which was protected as its ace ...
... Toward this end, debromination of 57 was effected by treatment with ntributyltin hydride in refluxing benzene in the presence of cat. amounts of AIBN to furnish acetonide 58. Deprotection of the acetonide using cat. CSA in methanol proceeded cleanly to yield alcohol 59 which was protected as its ace ...
5 organic chemistry: functional groups
... There is no change in the number of valence electrons on any of the atoms in the reaction. Both before and after the reaction, each carbon atom shares a total of eight valence electrons and each hydrogen atom shares two electrons. Instead of electrons, the reaction involves the transfer of atoms—in ...
... There is no change in the number of valence electrons on any of the atoms in the reaction. Both before and after the reaction, each carbon atom shares a total of eight valence electrons and each hydrogen atom shares two electrons. Instead of electrons, the reaction involves the transfer of atoms—in ...
Chapter 1 Structure and Bonding
... 2) No Hydrogen Bonding is possible in R—O—R 3) Boiling Points are much lower than alcohols, more like haloalkanes 4) Water solubility much less than alcohols a) MeOMe and EtOEt have some water solubility b) Larger ethers are insoluble, very much like alkanes 5) Fairly unreactive, nonpolar solvents f ...
... 2) No Hydrogen Bonding is possible in R—O—R 3) Boiling Points are much lower than alcohols, more like haloalkanes 4) Water solubility much less than alcohols a) MeOMe and EtOEt have some water solubility b) Larger ethers are insoluble, very much like alkanes 5) Fairly unreactive, nonpolar solvents f ...
Investigation 3
... to show an even stronger positive inductive effect as there are two methyl groups bonded to the C bonded to the O in the hydroxyl group. 2. The procedure For checking the validity of my hypothesis I used molecular modeling. This tool allows producing the actual Electrostatic potential versus Electro ...
... to show an even stronger positive inductive effect as there are two methyl groups bonded to the C bonded to the O in the hydroxyl group. 2. The procedure For checking the validity of my hypothesis I used molecular modeling. This tool allows producing the actual Electrostatic potential versus Electro ...
2006 Practice Final Exam - Department of Chemistry | Oregon State
... is cis-[CuCl3F3]4-. is trans-[CuCl3F3]4-. is fac-[CuCl3F3]4-. is mer-[CuCl3F3]4-. is world-cup-fever-[CuCl3F3]4-. ...
... is cis-[CuCl3F3]4-. is trans-[CuCl3F3]4-. is fac-[CuCl3F3]4-. is mer-[CuCl3F3]4-. is world-cup-fever-[CuCl3F3]4-. ...
OChem 1 Mechanism Flashcards Dr. Peter Norris, 2015
... Acid-Base reactions are generally very fast (proton, H, is accessible) ...
... Acid-Base reactions are generally very fast (proton, H, is accessible) ...
A Model for Catalytically Active Zinc(I1) Ion in Liver
... (s), 938 (s), 874 (s) 766, 656 (s), 579, 523 cm-I. Anal. Calcd for CloH24N4Zn(CF3S03)2: C, 25.56; H, 4.29; N, 9.94. Found: C, 25.53; H, 4.34; N , 9.97. Reactions of p -Nitrobenzaldehyde (9) with Alcohols Catalyzed by Various Zn" Species. p-Nitrobenzaldehyde (9,0.125 mmol) was added in one portion to ...
... (s), 938 (s), 874 (s) 766, 656 (s), 579, 523 cm-I. Anal. Calcd for CloH24N4Zn(CF3S03)2: C, 25.56; H, 4.29; N, 9.94. Found: C, 25.53; H, 4.34; N , 9.97. Reactions of p -Nitrobenzaldehyde (9) with Alcohols Catalyzed by Various Zn" Species. p-Nitrobenzaldehyde (9,0.125 mmol) was added in one portion to ...
Ch.17Outline_001
... Polyfunctional acids - contain other functional groups Priority for naming compounds (by functional group) 1.Carboxyl 4. Alkene 2.Carbonyl 5. Alkyne a)Aldehyde 6. Alkoxy b)Ketone 7. Alkyl 3.Alcohol 8. Halogen Common P.A.s: Unsaturated (with double bond) Hydroxyl (with -OH group) Keto (with carbonyl ...
... Polyfunctional acids - contain other functional groups Priority for naming compounds (by functional group) 1.Carboxyl 4. Alkene 2.Carbonyl 5. Alkyne a)Aldehyde 6. Alkoxy b)Ketone 7. Alkyl 3.Alcohol 8. Halogen Common P.A.s: Unsaturated (with double bond) Hydroxyl (with -OH group) Keto (with carbonyl ...
Ch.17
... Polyfunctional acids - contain other functional groups Priority for naming compounds (by functional group) 1. Carboxyl 5. Alkene 2. Carbonyl 6. Alkyne a)Aldehyde 7. Alkoxy (ether) b)Ketone 8. Alkyl 3. Alcohol 9. Halogen 4. Amine* ...
... Polyfunctional acids - contain other functional groups Priority for naming compounds (by functional group) 1. Carboxyl 5. Alkene 2. Carbonyl 6. Alkyne a)Aldehyde 7. Alkoxy (ether) b)Ketone 8. Alkyl 3. Alcohol 9. Halogen 4. Amine* ...
Camp 1
... Carboxylic acid: a compound containing a COOH (carboxyl: carbonyl + hydroxyl) group • in a condensed structural formula, a carboxyl group may also be written -CO2H. O O CH3 COH ...
... Carboxylic acid: a compound containing a COOH (carboxyl: carbonyl + hydroxyl) group • in a condensed structural formula, a carboxyl group may also be written -CO2H. O O CH3 COH ...
CHAPTER 12 Solid-Phase Synthesis of Peptides Containing the
... numerous peptide-receptor systems. Systematic side-chain replacement is often the first step in the design process of higher-affinity ligands. Modification of the peptide backbone is another step in the design process, but requires more information about the stability and structure of the peptide. T ...
... numerous peptide-receptor systems. Systematic side-chain replacement is often the first step in the design process of higher-affinity ligands. Modification of the peptide backbone is another step in the design process, but requires more information about the stability and structure of the peptide. T ...
Reactions hydroxyl groups part-I
... Remember anomeric center acts as an alcohol or aldehyde Describe the three types of hydroxyl groups found in sugars Realize the importance of protec,on to allow regioselec,ve rxns of those unprotected Ex ...
... Remember anomeric center acts as an alcohol or aldehyde Describe the three types of hydroxyl groups found in sugars Realize the importance of protec,on to allow regioselec,ve rxns of those unprotected Ex ...
Solvothermal Synthesis of Polyazomethine Microspheres
... S2. Synthesis of 5, 10, 15, 20-Tetrakis(4-aminophenyl)-21H,23H-porphine,TAPR 5,10,15,20-tetrakis(4-nitrophenyl)-21H,23H-porphine was synthesised according to the previously reported procedure[S2] with a little modified. 4-nitrobenzaldehydewas(10.0 g,66mmol) dissolved in 60 mL nitrobenzene, to which ...
... S2. Synthesis of 5, 10, 15, 20-Tetrakis(4-aminophenyl)-21H,23H-porphine,TAPR 5,10,15,20-tetrakis(4-nitrophenyl)-21H,23H-porphine was synthesised according to the previously reported procedure[S2] with a little modified. 4-nitrobenzaldehydewas(10.0 g,66mmol) dissolved in 60 mL nitrobenzene, to which ...
Chapter 16: Carboxylic Acids, Esters, and Other Acid Derivatives
... compound. Since this sodium salt of propanoic acid - so start from propanoic acid is a three carbon acid with no carbon-carbon double bonds and this negative ion without the H+ atom is called propanoate. There this salt is named: sodium propanoate When the carboxylic acids form salts, the hydrogen i ...
... compound. Since this sodium salt of propanoic acid - so start from propanoic acid is a three carbon acid with no carbon-carbon double bonds and this negative ion without the H+ atom is called propanoate. There this salt is named: sodium propanoate When the carboxylic acids form salts, the hydrogen i ...
Chapter 20 Carboxylic Acids
... NH4+ are soluble in water. • Soap is the soluble sodium salt of a long chain fatty acid. • Salts can be formed by the reaction of an acid with NaHCO3, releasing CO2. ...
... NH4+ are soluble in water. • Soap is the soluble sodium salt of a long chain fatty acid. • Salts can be formed by the reaction of an acid with NaHCO3, releasing CO2. ...
Activation of Alcohols Toward Nucleophilic Substitution: Conversion
... alcohols are converted to saturated alkyl halides.6 Because the use of HCl shows poor results for the conversion of an alcohol to an alkyl chloride, a catalyst such as the zinc used in the Lucas reagent is required. This reaction was improved by adding zinc chloride and had the advantage of milder c ...
... alcohols are converted to saturated alkyl halides.6 Because the use of HCl shows poor results for the conversion of an alcohol to an alkyl chloride, a catalyst such as the zinc used in the Lucas reagent is required. This reaction was improved by adding zinc chloride and had the advantage of milder c ...
Chapter 21 aldehydes and ketones
... carbonyl carbon, but the “1” is usually omitted from the name. The ring is then numbered clockwise or counterclockwise to give the first substituent the lower number. ...
... carbonyl carbon, but the “1” is usually omitted from the name. The ring is then numbered clockwise or counterclockwise to give the first substituent the lower number. ...
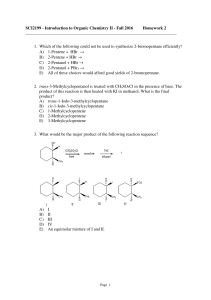
Document
... Addition of Br2 and Cl2 to Alkenes (Section 8.12) Stereochemistry of Dihalide Additions (Section 8.13) Halohydrin Formation [Net Addition of X-OH] (Section 8.14) Divalent Carbon Compounds: Carbenes (Section 8.15) Oxidations of Alkenes (Sections 8.16-8.17) Additions to Alkynes (Sections 8.18-8.19) Ox ...
... Addition of Br2 and Cl2 to Alkenes (Section 8.12) Stereochemistry of Dihalide Additions (Section 8.13) Halohydrin Formation [Net Addition of X-OH] (Section 8.14) Divalent Carbon Compounds: Carbenes (Section 8.15) Oxidations of Alkenes (Sections 8.16-8.17) Additions to Alkynes (Sections 8.18-8.19) Ox ...
Alcohol

In chemistry, an alcohol is any organic compound in which the hydroxyl functional group (–OH) is bound to a saturated carbon atom. The term alcohol originally referred to the primary alcohol ethyl alcohol (ethanol), the predominant alcohol in alcoholic beverages.The suffix -ol appears in the IUPAC chemical name of all substances where the hydroxyl group is the functional group with the highest priority; in substances where a higher priority group is present the prefix hydroxy- will appear in the IUPAC name. The suffix -ol in non-systematic names (such as paracetamol or cholesterol) also typically indicates that the substance includes a hydroxyl functional group and, so, can be termed an alcohol. But many substances, particularly sugars (examples glucose and sucrose) contain hydroxyl functional groups without using the suffix. An important class of alcohols, of which methanol and ethanol are the simplest members is the saturated straight chain alcohols, the general formula for which is CnH2n+1OH.