Alcohols phenols
... alcohols are covalent compounds, while inorganic hydroxides are ionic, (ii) alcohols do not ionize in water and are neutral to litmus, while inorganic hydroxides ionize and are alkaline towards litmus, (iii) alcohols undergo molecular reactions while inorganic hydroxides, ionic reactions. ...
... alcohols are covalent compounds, while inorganic hydroxides are ionic, (ii) alcohols do not ionize in water and are neutral to litmus, while inorganic hydroxides ionize and are alkaline towards litmus, (iii) alcohols undergo molecular reactions while inorganic hydroxides, ionic reactions. ...
Full Text - Verlag der Zeitschrift für Naturforschung
... In our previous research with carrot cell culture, its ability to perform the transformation of esters, alcohols and ketones was observed (Mironowicz and Kromer, 1998). Presently, the application of homogenized carrot root tissue, instead of the suspension cell culture, resulted in ca. 18 times high ...
... In our previous research with carrot cell culture, its ability to perform the transformation of esters, alcohols and ketones was observed (Mironowicz and Kromer, 1998). Presently, the application of homogenized carrot root tissue, instead of the suspension cell culture, resulted in ca. 18 times high ...
Chapter 8. CARBONYL COMPOUNDS
... The aldol can be easily dehydrated on heating because it retains the acidic α-hydrogen. α,βUnsaturated aldehydes and ketones are formed when the reaction is performed at elevated temperature. Crotonaldehyde is obtained from the simplest aldol in this way, therefore reactions of this type are called ...
... The aldol can be easily dehydrated on heating because it retains the acidic α-hydrogen. α,βUnsaturated aldehydes and ketones are formed when the reaction is performed at elevated temperature. Crotonaldehyde is obtained from the simplest aldol in this way, therefore reactions of this type are called ...
Review
... that Br2 would. Also unlike Br2, it goes only for the benzylic/allylic position, and doesn’t react significantly with tertiary carbons. Again, if you do this reaction at the allylic position, a mixture of products is possible based on different resonance forms. Reactions with Carbanions Again, a neg ...
... that Br2 would. Also unlike Br2, it goes only for the benzylic/allylic position, and doesn’t react significantly with tertiary carbons. Again, if you do this reaction at the allylic position, a mixture of products is possible based on different resonance forms. Reactions with Carbanions Again, a neg ...
Reactions of Alkenes and Alkynes
... intercepted by water nucleophile • Oxygen loses proton to give the neutral halohydrin product ...
... intercepted by water nucleophile • Oxygen loses proton to give the neutral halohydrin product ...
SBI4U: Biochemistry Functional Groups Activity Molecular Shape
... c) Why is one of the carbons is only linked to three other atoms? ______________________________________________________ d) Is this a polar or a non-polar molecule in solution? Explain. ____________________________________________________________ 3. Urea (CH4N2O). When proteins are digested and burn ...
... c) Why is one of the carbons is only linked to three other atoms? ______________________________________________________ d) Is this a polar or a non-polar molecule in solution? Explain. ____________________________________________________________ 3. Urea (CH4N2O). When proteins are digested and burn ...
TOPIC 6. NUCLEOPHILIC SUBSTITUTIONS (chapter 6 and parts of
... be classified as one of two types, based on these experimental observations. In order to develop predictive tools, we need to understand reasons why these observations are important. That is, we need to develop proposals for two different mechanisms which are consistent with the two sets of data and ...
... be classified as one of two types, based on these experimental observations. In order to develop predictive tools, we need to understand reasons why these observations are important. That is, we need to develop proposals for two different mechanisms which are consistent with the two sets of data and ...
Lab 9
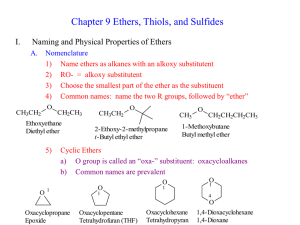
... Figure 3. Aliphatic or dialkyl ethers. Note: IUPAC name above and common name in blue. Aromatic Ethers Aromatic ethers are named in the same way as aliphatic ethers, except that the longer chain is generally an aromatic ring. Many aromatic ethers have common names. For example, methoxybenzene is ani ...
... Figure 3. Aliphatic or dialkyl ethers. Note: IUPAC name above and common name in blue. Aromatic Ethers Aromatic ethers are named in the same way as aliphatic ethers, except that the longer chain is generally an aromatic ring. Many aromatic ethers have common names. For example, methoxybenzene is ani ...
Chapter 8 Lecture
... Alkyl sulfonates (ROTs) are prepared by reaction of alcohols with a sulfonyl chloride: ...
... Alkyl sulfonates (ROTs) are prepared by reaction of alcohols with a sulfonyl chloride: ...
More reactions of alkenes Objective
... double bond and produce a diol • understand that heterolytic bond fission of a covalent bond results in the formation of ions • understand the mechanism of the electrophilic addition reactions for the reaction of alkenes with halogens including using curly arrow notation and other given binary compo ...
... double bond and produce a diol • understand that heterolytic bond fission of a covalent bond results in the formation of ions • understand the mechanism of the electrophilic addition reactions for the reaction of alkenes with halogens including using curly arrow notation and other given binary compo ...
Named Reactions Of Haloalkanes and haloarenes
... 3)Friedel craft’s reaction In this reaction, benzene is treated with alkyl halide or acyl chloride in the presence of anhydrous aluminium chloride acting as a catalyst. As a result,a hydrogen atom in the ring gets replaced either by alkyl group or acyl group. ...
... 3)Friedel craft’s reaction In this reaction, benzene is treated with alkyl halide or acyl chloride in the presence of anhydrous aluminium chloride acting as a catalyst. As a result,a hydrogen atom in the ring gets replaced either by alkyl group or acyl group. ...
Ch 9 Lecture 2
... 2) No Hydrogen Bonding is possible in R—O—R 3) Boiling Points are much lower than alcohols, more like haloalkanes 4) Water solubility much less than alcohols a) MeOMe and EtOEt have some water solubility b) Larger ethers are insoluble, very much like alkanes 5) Fairly unreactive, nonpolar solvents f ...
... 2) No Hydrogen Bonding is possible in R—O—R 3) Boiling Points are much lower than alcohols, more like haloalkanes 4) Water solubility much less than alcohols a) MeOMe and EtOEt have some water solubility b) Larger ethers are insoluble, very much like alkanes 5) Fairly unreactive, nonpolar solvents f ...
CHEM 121: chapter 16
... Alkanoic anhydride (-oic anhydride suffix) E.g. anhydride of proponoic acid is named as proponoic anhydride ...
... Alkanoic anhydride (-oic anhydride suffix) E.g. anhydride of proponoic acid is named as proponoic anhydride ...
The Baylis–Hillman reaction is an organic reaction of an aldehyde
... The Henry Reaction is a base-catalyzed C-C bond-forming reaction between nitroalkanes and aldehydes or ketones. It is similar to the Aldol Addition, and also referred to as the Nitro Aldol Reaction. If acidic protons are available (i.e. when R = H), the products tend to eliminate water to give nitr ...
... The Henry Reaction is a base-catalyzed C-C bond-forming reaction between nitroalkanes and aldehydes or ketones. It is similar to the Aldol Addition, and also referred to as the Nitro Aldol Reaction. If acidic protons are available (i.e. when R = H), the products tend to eliminate water to give nitr ...
Document
... • Is named by selecting the longest carbon chain that contain the -SH. We add -thiol to the name of the parent alkane. • Parent chain is numbered from the end nearest to the -SH group. CH3─S─H ...
... • Is named by selecting the longest carbon chain that contain the -SH. We add -thiol to the name of the parent alkane. • Parent chain is numbered from the end nearest to the -SH group. CH3─S─H ...
1 Carbonyl Condensation Reactions (Conjugate Addition) If we look
... We’ve already seen these are used to prepare ketones from acid chlorides – formation of copper salts is a great way to “soften” a hard nucleophile. Thus, “soft” nucleophiles favor conjugate addition to α,β-unsaturated compounds. Hence non-carbon nucleophiles like nitrogen (amines) and oxygen (alcoho ...
... We’ve already seen these are used to prepare ketones from acid chlorides – formation of copper salts is a great way to “soften” a hard nucleophile. Thus, “soft” nucleophiles favor conjugate addition to α,β-unsaturated compounds. Hence non-carbon nucleophiles like nitrogen (amines) and oxygen (alcoho ...
Retrosynthesis - Organic Chemistry
... • the FIRST REACTION WORKING BACKWARDS (the last reaction forwards) must be in the has an alkene as the product • alkenes can be formed by elimination from halides or alcohols • of these two we choose the halide reaction, because the halide can be made from the starting structure more easily than th ...
... • the FIRST REACTION WORKING BACKWARDS (the last reaction forwards) must be in the has an alkene as the product • alkenes can be formed by elimination from halides or alcohols • of these two we choose the halide reaction, because the halide can be made from the starting structure more easily than th ...
Chemistry 209 - Experiment 3, Fall 2002
... Water Solubility Test. The general rule for solubility is "like dissolves like". Hence water, a very polar solvent, dissolves only the most polar organic compounds, for example, salts of organic acids, salts of organic bases, lower molecular weight alcohols and amines (e.g., ethanol, methylamine), a ...
... Water Solubility Test. The general rule for solubility is "like dissolves like". Hence water, a very polar solvent, dissolves only the most polar organic compounds, for example, salts of organic acids, salts of organic bases, lower molecular weight alcohols and amines (e.g., ethanol, methylamine), a ...
Chemdraw B&W - Pennsylvania State University
... • SN2 reaction:, the leaving group X can be chloride, bromide, iodide, or tosylate • R should be primary or methyl and preferably should be allylic or benzylic • Secondary halides react poorly, and tertiary halides don't react at all because of competing elimination ...
... • SN2 reaction:, the leaving group X can be chloride, bromide, iodide, or tosylate • R should be primary or methyl and preferably should be allylic or benzylic • Secondary halides react poorly, and tertiary halides don't react at all because of competing elimination ...
Document
... LESS THAN FOUR attachments. – Alkenes and alkynes are unsaturated. – They contain at least one double or triple bond, respectively. – They have fewer hydrogen atoms per carbon atom than alkanes. © 2013 Pearson Education, Inc. ...
... LESS THAN FOUR attachments. – Alkenes and alkynes are unsaturated. – They contain at least one double or triple bond, respectively. – They have fewer hydrogen atoms per carbon atom than alkanes. © 2013 Pearson Education, Inc. ...
Alcohol

In chemistry, an alcohol is any organic compound in which the hydroxyl functional group (–OH) is bound to a saturated carbon atom. The term alcohol originally referred to the primary alcohol ethyl alcohol (ethanol), the predominant alcohol in alcoholic beverages.The suffix -ol appears in the IUPAC chemical name of all substances where the hydroxyl group is the functional group with the highest priority; in substances where a higher priority group is present the prefix hydroxy- will appear in the IUPAC name. The suffix -ol in non-systematic names (such as paracetamol or cholesterol) also typically indicates that the substance includes a hydroxyl functional group and, so, can be termed an alcohol. But many substances, particularly sugars (examples glucose and sucrose) contain hydroxyl functional groups without using the suffix. An important class of alcohols, of which methanol and ethanol are the simplest members is the saturated straight chain alcohols, the general formula for which is CnH2n+1OH.