
fahad h. ahmad - Fahad`s Academy
... Where x = distance moved by the substance and; y = distance moved by the solvent Checking the Purity of Substances - Pure substances have FIXED MELTING AND BOILING POINTS. Pure water boils at 100oC and melts at 0oC. - Impure substances have NO FIXED MELTING AND BOILING POINTS. They melt and boil a ...
... Where x = distance moved by the substance and; y = distance moved by the solvent Checking the Purity of Substances - Pure substances have FIXED MELTING AND BOILING POINTS. Pure water boils at 100oC and melts at 0oC. - Impure substances have NO FIXED MELTING AND BOILING POINTS. They melt and boil a ...
INTRODUCING ACYL CHLORIDES (acid
... group in the acid is replaced by something else. Compounds like this are described as acid derivatives. Acyl chlorides (also known as acid chlorides) are one example of an acid derivative. In this case, the -OH group has been replaced by a chlorine atom. ...
... group in the acid is replaced by something else. Compounds like this are described as acid derivatives. Acyl chlorides (also known as acid chlorides) are one example of an acid derivative. In this case, the -OH group has been replaced by a chlorine atom. ...
Chemistry in Society - Cathkin High School
... They must take account of cost of raw materials, plant-running costs etc. If the yield of product is not sufficient enough to cover the costs of production then the process would not be considered to be economically viable. ...
... They must take account of cost of raw materials, plant-running costs etc. If the yield of product is not sufficient enough to cover the costs of production then the process would not be considered to be economically viable. ...
1 - University of Missouri
... 1. Propose the starting materials needed to prepare the following compounds by the Grignard reaction (in pen), using phenylmagnesium bromide as the Grignard Reagent. ...
... 1. Propose the starting materials needed to prepare the following compounds by the Grignard reaction (in pen), using phenylmagnesium bromide as the Grignard Reagent. ...
p Block Elements General Configuration: ns2 np1
... Nitrogen differs from the rest of the members of this group due to its smaller size, high electro negativity, high ionization enthalpy and non-availability of d-orbitals. Nitrogen can form pπ-pπ multiple bond. Nitrogen exists as diatomic molecule with a triple bond. Heavier elements do not form pπ-p ...
... Nitrogen differs from the rest of the members of this group due to its smaller size, high electro negativity, high ionization enthalpy and non-availability of d-orbitals. Nitrogen can form pπ-pπ multiple bond. Nitrogen exists as diatomic molecule with a triple bond. Heavier elements do not form pπ-p ...
Midterm Exam March NAME
... preparation of viagra. Show how you would synthesis this trisubstituted derivative starting from benzene and any other reagents you need. Hint - you should be able to do this in five steps. Start by ...
... preparation of viagra. Show how you would synthesis this trisubstituted derivative starting from benzene and any other reagents you need. Hint - you should be able to do this in five steps. Start by ...
INTRODUCING PHENOL
... However, for compounds containing benzene rings, combustion is hardly ever complete, especially if they are burnt in air. The high proportion of carbon in phenol means that you need a very high proportion of oxygen to phenol to get complete combustion. Look at the equation. As a general rule, the hy ...
... However, for compounds containing benzene rings, combustion is hardly ever complete, especially if they are burnt in air. The high proportion of carbon in phenol means that you need a very high proportion of oxygen to phenol to get complete combustion. Look at the equation. As a general rule, the hy ...
1. (a) Propan-1ol, C2H5CH2OH can be oxidised to propanoic acid
... Benzocaine, C9H11O2N, is an aromatic compound which is used commercially in creams to alleviate sunburn. Benzocaine reacts with dilute acids to form the ion C9H12O2N+ and with ethanoyl chloride to form C11H13O3N. When benzocaine is heated under reflux with aqueous sodium hydroxide and the soluti ...
... Benzocaine, C9H11O2N, is an aromatic compound which is used commercially in creams to alleviate sunburn. Benzocaine reacts with dilute acids to form the ion C9H12O2N+ and with ethanoyl chloride to form C11H13O3N. When benzocaine is heated under reflux with aqueous sodium hydroxide and the soluti ...
S11 HMP - ISpatula
... The oxidative portion of the HMP leads to the formation of ribulose 5phosphate, CO2, and two molecules of NADPH for each molecule of glucose 6-phosphate oxidized. A. Dehydrogenation of glucose 6-phosphate B. Hydrolysis of 6-phosphogluconolactone and formation of ribulose 5phosphate. ...
... The oxidative portion of the HMP leads to the formation of ribulose 5phosphate, CO2, and two molecules of NADPH for each molecule of glucose 6-phosphate oxidized. A. Dehydrogenation of glucose 6-phosphate B. Hydrolysis of 6-phosphogluconolactone and formation of ribulose 5phosphate. ...
top organomet chem-2006-19-207 pauson
... (Scheme 4) [25]. Substitution at the double bond is restricted as disubstituted olefins usually fail to react. The idea that alkenes possessing electron-withdrawing groups are not adequate substrates for Pauson–Khand reactions has in recent years turned out not to be precise. Carretero has reported s ...
... (Scheme 4) [25]. Substitution at the double bond is restricted as disubstituted olefins usually fail to react. The idea that alkenes possessing electron-withdrawing groups are not adequate substrates for Pauson–Khand reactions has in recent years turned out not to be precise. Carretero has reported s ...
Chapter 15: Kinetics
... The table below presents plote of concentration of biologically active metabolite T-IDA vs time. Using of these data determine graphically the half-time life (t1/2) of this metabolite. ...
... The table below presents plote of concentration of biologically active metabolite T-IDA vs time. Using of these data determine graphically the half-time life (t1/2) of this metabolite. ...
Energetics - WordPress.com
... different bonds with different strengths are being broken and formed. The amount (or concentration) of reactants; the greater the amount that reacts , the greater the heat change. The states of the reactants and products – changing state involves an enthalpy change, and so will affect the total amou ...
... different bonds with different strengths are being broken and formed. The amount (or concentration) of reactants; the greater the amount that reacts , the greater the heat change. The states of the reactants and products – changing state involves an enthalpy change, and so will affect the total amou ...
Revised Syllabus - M. Sc. First Year - Chemistry
... a) Types of Aliphatic electrophilic substitution reaction: SE1, SE2, SEi. b) Electrophilic substitution accompanied by double bond shift. c) Effect of substrate, leaving group and solvent polarity on the reactivity. ...
... a) Types of Aliphatic electrophilic substitution reaction: SE1, SE2, SEi. b) Electrophilic substitution accompanied by double bond shift. c) Effect of substrate, leaving group and solvent polarity on the reactivity. ...
answers to part a of the national high school
... when solving this type of problem. Note also that the oxide U3O8 must first be converted into UO2 before it can be used in CANDU reactors. 4. This was another question that students in most (but not all) provinces found easy, but its discrimination index was very poor. About 72 % of teachers felt th ...
... when solving this type of problem. Note also that the oxide U3O8 must first be converted into UO2 before it can be used in CANDU reactors. 4. This was another question that students in most (but not all) provinces found easy, but its discrimination index was very poor. About 72 % of teachers felt th ...
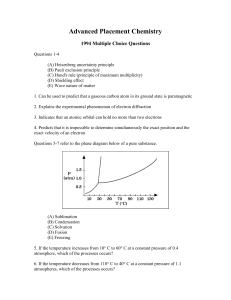
Advanced Placement Chemistry
... III. Addition of some base beyond the equivalence point (A) I only (B) III only (C) I and II only (D) II and III only (E) I, II, and III 71 ... Fe(OH)2 + ... O2 + ... H2O ---> ... Fe(OH)3 If 1 mole of O2 oxidizes Fe(OH)2 according to the reaction represented above, how many moles of Fe(OH)3 can be f ...
... III. Addition of some base beyond the equivalence point (A) I only (B) III only (C) I and II only (D) II and III only (E) I, II, and III 71 ... Fe(OH)2 + ... O2 + ... H2O ---> ... Fe(OH)3 If 1 mole of O2 oxidizes Fe(OH)2 according to the reaction represented above, how many moles of Fe(OH)3 can be f ...
Alcohols and Phenols
... Alcohols and Phenols Alcohols contain an OH group connected to a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to a carbon in a benzene ...
... Alcohols and Phenols Alcohols contain an OH group connected to a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to a carbon in a benzene ...
Mock-UP - GEOCITIES.ws
... Because the carbonyl and hydroxyl groups are directly attached to one another, there is a strong resonance interaction between the two groups, which sharply modifies the properties of the compound functionality from that of either of the two component functionalities. What is the reason for the much ...
... Because the carbonyl and hydroxyl groups are directly attached to one another, there is a strong resonance interaction between the two groups, which sharply modifies the properties of the compound functionality from that of either of the two component functionalities. What is the reason for the much ...
введение в общую introductio to the general ch ведение в общую
... Chemical properties of a substance are described as its abilities to form other substances in different conditions. In physical processes a substance changes at least one of its conditions: its volume, its shape, its position in the space, etc., while new substances are not formed. Phase transitions ...
... Chemical properties of a substance are described as its abilities to form other substances in different conditions. In physical processes a substance changes at least one of its conditions: its volume, its shape, its position in the space, etc., while new substances are not formed. Phase transitions ...
Chapter Seventeen
... Phosphate ester: A compound formed by reaction of an alcohol with phosphoric acid; may be a monoester, a diester, or a triester, also may be a di- or triphosphate. ...
... Phosphate ester: A compound formed by reaction of an alcohol with phosphoric acid; may be a monoester, a diester, or a triester, also may be a di- or triphosphate. ...
ChemChapter_7sec1_and_section2[1]FORMULA
... 1. The oxidation number of an element in its elemental form is zero. Examples of this are N2 (g), O2 (g), Na (s), Cl2 (g), etc. 2. The oxidation number of a monatomic ion is exactly the same as its charge. So, Group IA ions will all have an oxidation number of +1, since they all lose one electron. G ...
... 1. The oxidation number of an element in its elemental form is zero. Examples of this are N2 (g), O2 (g), Na (s), Cl2 (g), etc. 2. The oxidation number of a monatomic ion is exactly the same as its charge. So, Group IA ions will all have an oxidation number of +1, since they all lose one electron. G ...
Ordinary Level - State Examination Commission
... Why is electrical power transmitted at high voltages over long distances? ...
... Why is electrical power transmitted at high voltages over long distances? ...
Strychnine total synthesis

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field. At the time it formed the natural conclusion to an elaborate process of molecular structure elucidation that started with the isolation of strychnine from the beans of Strychnos ignatii by Pierre Joseph Pelletier and Joseph Bienaimé Caventou in 1818. Major contributors to the entire effort were Sir Robert Robinson with over 250 publications and Hermann Leuchs with another 125 papers in a time span of 40 years. Robinson was awarded the Nobel Prize in Chemistry in 1947 for his work on alkaloids, strychnine included. The process of chemical identification was completed with publications in 1946 by Robinson and later confirmed by Woodward in 1947. X-ray structures establishing the absolute configuration became available between 1947 and 1951 with publications from J. M. Bijvoet and J.H. Robertson .Woodward published a very brief account on the strychnine synthesis in 1954 (just 3 pages) and a lengthy one (42 pages) in 1963.Many more methods exist and reported by the research groups of Magnus, Overman, Kuehne, Rawal, Bosch, Vollhardt, Mori, Shibasaki, Li, Fukuyama Vanderwal and MacMillan. Synthetic (+)-strychnine is also known. Racemic synthesises were published by Padwa in 2007 and in 2010 by Andrade and by Reissig.In his 1963 publication Woodward quoted Sir Robert Robinson who said for its molecular size it is the most complex substance known.





















![ChemChapter_7sec1_and_section2[1]FORMULA](http://s1.studyres.com/store/data/000546743_1-278f96ccbbfd49e292510ec017e27124-300x300.png)
