`A` LEVEL H2 CHEMISTRY ORGANIC REACTIONS SUMMARY By
... CO2 (linear); CH4 (tetrahedral); NH3 (trigonal pyramidal); H2O (non-linear); SF6 (octahedral) by using the Valence Shell Electron Pair Repulsion theory (d) describe covalent bonding in terms of orbital overlap, giving σ and π bonds (see also ...
... CO2 (linear); CH4 (tetrahedral); NH3 (trigonal pyramidal); H2O (non-linear); SF6 (octahedral) by using the Valence Shell Electron Pair Repulsion theory (d) describe covalent bonding in terms of orbital overlap, giving σ and π bonds (see also ...
Chemistry 1B General Chemistry Laboratory
... you may not be allowed to work. Table of Contents: You must leave several pages blank at the beginning of your notebook for table of contents. That table will list each experiment that has been done and the page number for which it can be found. Experimental Notes and Content: • Title Page: Each exp ...
... you may not be allowed to work. Table of Contents: You must leave several pages blank at the beginning of your notebook for table of contents. That table will list each experiment that has been done and the page number for which it can be found. Experimental Notes and Content: • Title Page: Each exp ...
First palladium- and nickel-catalyzed oxidative
... accomplished. Related uses of transition-metal promoters such as thallium [7a], mercury [7b,c], palladium [7d,e], or copper [7f,g] also require stoichiometric amounts due to related formation of stable transition metal-diamine complexes or metal reoxidation incompatibility. The drawback of stoichiom ...
... accomplished. Related uses of transition-metal promoters such as thallium [7a], mercury [7b,c], palladium [7d,e], or copper [7f,g] also require stoichiometric amounts due to related formation of stable transition metal-diamine complexes or metal reoxidation incompatibility. The drawback of stoichiom ...
Topic 17 specification content - A
... I can describe the nucleophilic addition–elimination reactions of water, alcohols, ammonia and primary amines with acyl chlorides and acid anhydrides and outline the mechanism of the nucleophilic addition–elimination reactions of acyl chlorides with water, alcohols, ammonia and primary amines ...
... I can describe the nucleophilic addition–elimination reactions of water, alcohols, ammonia and primary amines with acyl chlorides and acid anhydrides and outline the mechanism of the nucleophilic addition–elimination reactions of acyl chlorides with water, alcohols, ammonia and primary amines ...
Chapter 13: Water and the Lithosphere Preview
... Figure 13.1 Polymeric structure of silicon dioxide. Al2Si2O5(OH)4 is the formula for the secondary mineral kaolinite, a member of the clay mineral class. Although it has a specific structure [see p. ?], it can formally be considered as being made up of a mole of Al2O3, two moles of SiO2, and two mo ...
... Figure 13.1 Polymeric structure of silicon dioxide. Al2Si2O5(OH)4 is the formula for the secondary mineral kaolinite, a member of the clay mineral class. Although it has a specific structure [see p. ?], it can formally be considered as being made up of a mole of Al2O3, two moles of SiO2, and two mo ...
AP Chemistry Summer Assignment
... About the AP Chemistry Course: Since this is a college level course taught in high school, it is very demanding, both in time and effort required. Much of the work involves solving math-type story problems. Homework is assigned each day through all three trimesters. The three weeks before the AP Exa ...
... About the AP Chemistry Course: Since this is a college level course taught in high school, it is very demanding, both in time and effort required. Much of the work involves solving math-type story problems. Homework is assigned each day through all three trimesters. The three weeks before the AP Exa ...
Presentation
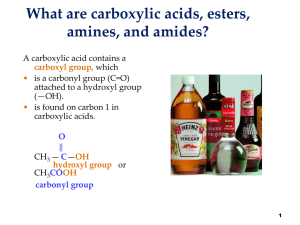
... or in carboxylic acids (CH3COOH) due to resonance stabilisation of carbanion. (weak base, its conjugate acid is strong) ...
... or in carboxylic acids (CH3COOH) due to resonance stabilisation of carbanion. (weak base, its conjugate acid is strong) ...
Synthesis of Heterocycles from Anthranilic acid
... acid tryptophan, as well as a catabolic product of tryptophan in animals. It is also integrated into many alkaloids isolated from plants. Aa is produced industrially for production of dyestuffs and pharmaceuticals. The dissertation gives a historical background and a short review on the reactivity o ...
... acid tryptophan, as well as a catabolic product of tryptophan in animals. It is also integrated into many alkaloids isolated from plants. Aa is produced industrially for production of dyestuffs and pharmaceuticals. The dissertation gives a historical background and a short review on the reactivity o ...
Stoichiometry Notes
... an unknown substance and the solute of the standard solution. The completion of the reaction is indicated by the end point of the reaction, which is observed by the colour change either due to the indicator or due to the solute itself. Whether the reactions during the analysis are either between an ...
... an unknown substance and the solute of the standard solution. The completion of the reaction is indicated by the end point of the reaction, which is observed by the colour change either due to the indicator or due to the solute itself. Whether the reactions during the analysis are either between an ...
Origins of Medicinal Chemistry
... 1880s- Kussmaul’s lab in Strasbourg is trying to eliminate intestinal worms with naphthalene- they are mistakenly given acetanilide, which proves to be an effective antipyretic Bayer & Co. makes ethoxyacetanilide and markets it as phenacetin ...
... 1880s- Kussmaul’s lab in Strasbourg is trying to eliminate intestinal worms with naphthalene- they are mistakenly given acetanilide, which proves to be an effective antipyretic Bayer & Co. makes ethoxyacetanilide and markets it as phenacetin ...
Organic Molecules
... structures containing thousands of atoms! Although carbon is present in all organic compounds, other elements such as hydrogen (H), oxygen (O), nitrogen (N), sulfur (S) and phosphorus (P) are also common in these molecules. Until the early nineteenth century, chemists had managed to make many simple ...
... structures containing thousands of atoms! Although carbon is present in all organic compounds, other elements such as hydrogen (H), oxygen (O), nitrogen (N), sulfur (S) and phosphorus (P) are also common in these molecules. Until the early nineteenth century, chemists had managed to make many simple ...
Chemistry 209 - Experiment 3, Fall 2002
... Cover the tube with a cork or rubber sleeve stopper and shake it vigorously so as to mix the contents thoroughly after each addition of solvent. If the substance dissolves completely, record it as soluble. Continue shaking for at least five minutes if the substance does not dissolve at first in orde ...
... Cover the tube with a cork or rubber sleeve stopper and shake it vigorously so as to mix the contents thoroughly after each addition of solvent. If the substance dissolves completely, record it as soluble. Continue shaking for at least five minutes if the substance does not dissolve at first in orde ...
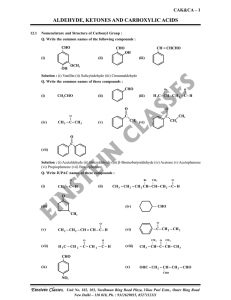
aldehyde, ketones and carboxylic acids
... intermediate captures a proton from the reaction medium to give the electrically neutral product. The net result is addition of Nu– and H+ across the carbon oxygen double bond. Q. Why Aldehydes are more reactive than Ketones ? Solution : There are two reasons for this, they are as follows : 1. Steri ...
... intermediate captures a proton from the reaction medium to give the electrically neutral product. The net result is addition of Nu– and H+ across the carbon oxygen double bond. Q. Why Aldehydes are more reactive than Ketones ? Solution : There are two reasons for this, they are as follows : 1. Steri ...
reactions of the conjugated dienes butadiene and isoprene alone
... more acidic than the N F +- and Co2+-exchanged materials (see, e.g., Adams et al., 1982b; Mortland, 1968). The fourth type of catalyst was a montmorillonite that contained a mixture of Ni z+ and Ag + as exchangeable cations. This catalyst was expected to show some acidity (derived from the dissociat ...
... more acidic than the N F +- and Co2+-exchanged materials (see, e.g., Adams et al., 1982b; Mortland, 1968). The fourth type of catalyst was a montmorillonite that contained a mixture of Ni z+ and Ag + as exchangeable cations. This catalyst was expected to show some acidity (derived from the dissociat ...
Chapter 14
... could do a Grignard reaction with methyl magnesium bromide and we could form our target molecule. But then the question in our minds is how do we make the six-carbon dialdehyde. Working backward one more step, we recall that we could form this using the ozonolysis reaction with a reductive work-up f ...
... could do a Grignard reaction with methyl magnesium bromide and we could form our target molecule. But then the question in our minds is how do we make the six-carbon dialdehyde. Working backward one more step, we recall that we could form this using the ozonolysis reaction with a reductive work-up f ...
chemistry paper 1
... cleaner was first diluted to 250.0 cm3 with distilled water. Upon titration with 0.950 M sodium hydroxide solution using phenolphthalein as indicator, 25.0 cm3 of the diluted cleaner required 27.1 cm3 of the sodium hydroxide solution to reach the end point. ...
... cleaner was first diluted to 250.0 cm3 with distilled water. Upon titration with 0.950 M sodium hydroxide solution using phenolphthalein as indicator, 25.0 cm3 of the diluted cleaner required 27.1 cm3 of the sodium hydroxide solution to reach the end point. ...
ALKANOLS (ALCOHOLS)
... The prefixes di and tri are used as required to indicate more than one hydroxyl or alkyl group Isomerism and Classification: Alcohols can exhibit structural isomerism due to: The presence and position of alkyl side chains The position of the hydroxyl group Alcohols can also exhibit optical iso ...
... The prefixes di and tri are used as required to indicate more than one hydroxyl or alkyl group Isomerism and Classification: Alcohols can exhibit structural isomerism due to: The presence and position of alkyl side chains The position of the hydroxyl group Alcohols can also exhibit optical iso ...
Strychnine total synthesis

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field. At the time it formed the natural conclusion to an elaborate process of molecular structure elucidation that started with the isolation of strychnine from the beans of Strychnos ignatii by Pierre Joseph Pelletier and Joseph Bienaimé Caventou in 1818. Major contributors to the entire effort were Sir Robert Robinson with over 250 publications and Hermann Leuchs with another 125 papers in a time span of 40 years. Robinson was awarded the Nobel Prize in Chemistry in 1947 for his work on alkaloids, strychnine included. The process of chemical identification was completed with publications in 1946 by Robinson and later confirmed by Woodward in 1947. X-ray structures establishing the absolute configuration became available between 1947 and 1951 with publications from J. M. Bijvoet and J.H. Robertson .Woodward published a very brief account on the strychnine synthesis in 1954 (just 3 pages) and a lengthy one (42 pages) in 1963.Many more methods exist and reported by the research groups of Magnus, Overman, Kuehne, Rawal, Bosch, Vollhardt, Mori, Shibasaki, Li, Fukuyama Vanderwal and MacMillan. Synthetic (+)-strychnine is also known. Racemic synthesises were published by Padwa in 2007 and in 2010 by Andrade and by Reissig.In his 1963 publication Woodward quoted Sir Robert Robinson who said for its molecular size it is the most complex substance known.