* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Functions of carbohydrates
Survey
Document related concepts
Transcript
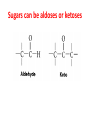
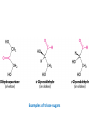
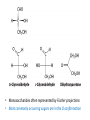
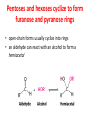
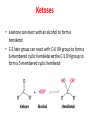
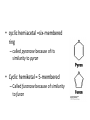
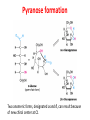
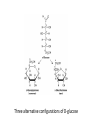
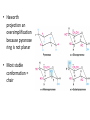
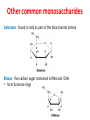
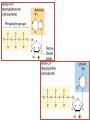
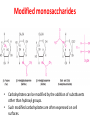
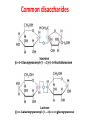
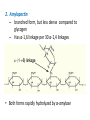
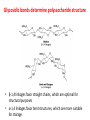
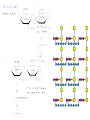
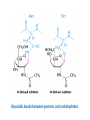
Functions of carbohydrates energy stores, fuels, and metabolic intermediates structural framework of RNA and DNA structural elements in the cell walls of bacteria and plants linked to many proteins and lipids Carbohydrates • aldehyde or ketone compounds with multiple hydroxyl groups • constructed of carbon (carbo-) plus hydrogen and oxygen (-hydrate) • simplest carbohydrates = monosaccharides, or simple sugars Cn(H2O)n • make up most of the organic matter on Earth • Starch consists of chains of linked glucose molecules. • These chains are broken down into individual glucose molecules • Glucose catabolism used to generate ATP and building blocks for other molecules. Sugars can be aldoses or ketoses D-aldoses • contain an aldehyde group • have Dglyceraldehyde at the asymmetric center farthest from the aldehyde group D-ketoses • have one fewer asymmetric center than aldoses with the same number of carbons • D-Fructose is the most abundant ketohexose Examples of triose sugars • Monosaccharides often represented by Fischer projections • Most commonly occurring sugars are in the D conformation 6 – carbon aldoses Epimers • Sugars that differ in a single chiral center • Examples: 1. Galactose and glucose (C-4 epimers) 2. Glucose and mannose (C-2 epimers) Pentoses and hexoses cyclize to form furanose and pyranose rings • open-chain forms usually cyclize into rings • an aldehyde can react with an alcohol to form a hemiacetal Ketoses • a ketone can react with an alcohol to form a hemiketal • C-2 keto group can react with C-6 OH group to form a 6-membered cyclic hemiketal or the C-5 OH group to form a 5-membered cyclic hemiketal • cyclic hemiacetal =six-membered ring – called pyranose because of its similarity to pyran • Cyclic hemiketal = 5-membered – Called furanose because of similarity to furan Pyranose formation Two anomeric forms, designated α and β, can result because of new chiral center at C1 Three alternative configurations of D-glucose • An equilibrium mixture of glucose contains ~1/3 α anomer 2/3 β anomer <1% of the open-chain form • Glucose stored by plants (starch) and animals (glycogen) • Also present in cellulose; provides structural integrity to the cells Most biologically important sugars are six-carbon hexoses that are structurally related to D-glucose • Haworth projection an oversimplification because pyranose ring is not planar • Most stable conformation = chair Furanose formation • open-chain form of fructose cyclizes to a 5-membered ring when the C-5 OH group attacks the C-2 ketone to form an intramolecular hemiketal • Two anomers are possible Fructose • forms both pyranose and furanose rings • pyranose form predominates in fructose free in solution • furanose form predominates in many fructose derivatives Other common monosaccharides Galactose - found in milk as part of the disaccharide lactose Ribose - five carbon sugar contained in RNA and DNA • form furanose rings Reactions of monosaccharides • Oxidation - provides E for organisms • Produces sugar acids • For hexoses: 1. Oxidation at C1 → aldonic acid Detected by Benedict’s, Barfoed’s or Seliwanoff’s test 2. Oxidation at C6 → uronic acid 3. Oxidation at C1 and C6 → aldaric acid Oxidation of sugars Sugars that react are called reducing sugars; those that do not are called nonreducing sugars Lactones • Produced from oxidation of aldose e.g., α-D-glucose → lactone in presence of silver-ammonia complex – basis of Tollen’s test – Positive result = silver mirror Glycoside formation • Glycoside = non-reducing sugar – C1 not free because linked with R group – Usually, -OH group bonded to anomeric C in cyclic form Hemiacetal + R – OH → full acetal/glycoside Anomeric carbon atom reacts with the hydroxyl group of methanol Exhaustive methylation • R-OH reactions only affect anomeric C’s • Other R groups can be methylated using dimethylsulfate – Used to determine presence of glycosidic linkages Reduction reactions • Formation of alditols – C=O to C – OH – E.g., sorbose → sorbitol Xylose → xylitol • Formation of sugar alcohols – C – OH to C – H – e.g., β-D-ribose → β-D-deoxyribose Esterification reactions • -OH groups of sugars behave exactly like all other alcohols • May reach with acids, acid derivatives, phosphates, etc • e.g., phosphates esterified to ribose or deoxyribose Modified monosaccharides • • Carbohydrates can be modified by the addition of substituents other than hydroxyl groups. Such modified carbohydrates are often expressed on cell surfaces. Formation of sugar sulfates • Sulfates esterified at C-2, C-4 or C-6 of aldoses • Found mostly in proteoglycans of ECM • Presence of sulfate groups mean that sugar is negatively charged at physiological pH The major glycosaminoglycans Polysaccharides Carbohydrates • aldehyde or ketone compounds with multiple OH groups • constructed of carbon (carbo-) plus hydrogen and oxygen (-hydrate) • simplest carbohydrates = monosaccharides, or simple sugars Cn(H2O)n • make up most of the organic matter on Earth Polysaccharides • play vital roles in energy storage, and in maintaining structural integrity of an organism • May be 1. Homopolysaccharides – all of the monosaccharides are the same – e.g., starch, glycogen 2. Heteropolysaccharides – >1 type of monosaccharide – Usually just 2 alternating monosaccharide units Complex carbohydrates formed by linkage of monosaccharides • Oligosaccharides formed by O-glycosidic linkages between monosaccharide units • Since monosaccharides have multiple OH groups, various glycosidic linkages are possible Glycosidic bonds • A large no. of different glycosidic bonds can be formed between 2 sugar residues • For example, glucose could be bonded to fructose by any of the following linkages: o α(1→1) o α(1→2) o α(1→3) o α(1→4) o α(1→6) o β(1→1) o β(1→2) o β(1→3) o β(1→4) o β(1→6) Common disaccharides Common disaccharides Starch • nutritional reservoir in plants; makes up >50% of carbohydrates ingested by humans • 2 forms: amylose and amylopectin 1. Amylose – unbranched – consists of glucose residues in α-1,4 linkage. 2. Amylopectin – branched form, but less dense compared to glycogen – Has α-1,6 linkage per 30 α-1,4 linkages • Both forms rapidly hydrolyzed by α-amylase Glycogen • Storage form of glucose in animals • Main chain: glucose linked by α-1,4glycosidic bonds • Branches: formed by α1,6-glycosidic bonds Cross section of a glycogen molecule Branch point in glycogen • 2 chains of glucose molecules joined by α-1,4-glycosidic bonds are linked by an α-1,6-glycosidic bond to create a branch point • α-1,6-glycosidic bond forms every 10 glucose units Cellulose • the other major polysaccharide of glucose in plants • one of the most abundant organic compounds in the biosphere • unbranched polymer of glucose residues joined by β1,4 linkages • serves a structural rather than a nutritional role – Mammals lack cellulases; cannot digest wood, vegetable fibers Glycosidic bonds determine polysaccharide structure • β-1,4 linkages favor straight chains, which are optimal for structural purposes • α-1,4 linkages favor bent structures, which are more suitable for storage. Chitin • long-chain polymer of a N-acetylglucosamine • main component of the exoskeletons of arthropods, such as crustaceans and insects Dextran • synthesized from sucrose by certain lactic-acid bacteria – e.g., Leuconostoc mesenteroides, Streptococcus mutans • Found in dental plaque • Similar to amylopectin – main chains formed by α(1→6) glycosidic linkages – side branches attached by α(1→3) or α(1→4) linkages Dextran: uses • used in eye drops as a lubricant • used to replace lost blood when replacement blood is not available • BUT must be used with caution; does not contain electrolytes • Present in certain IV fluids to 1. solubilise other factors, e.g., iron (=iron dextran). 2. Provide energy: digested into glucose and free water Peptidoglycan • Makes up bacterial cell walls • linear polysaccharide chains cross-linked by short peptides • mechanical support • prevents bacteria from bursting in response to their high internal osmotic pressure • Penicillin inhibits cross-linking transpeptidase Formation of crosslinks in peptidoglycan • catalyzed by glycopeptide transpeptidase • Penicillin mimics d-Ala-d-Ala moiety of normal substrate – Bound penicillin forms covalent bond with Ser at active site of enzyme – penicilloyl-enzyme does not react further Glycoproteins • proteins that contain oligosaccharide chains covalently attached to their polypeptide sidechains • sugars may be attached to 1. N in asparagine side chain (N-linkage) 2. O in the side chain of serine or threonine (Olinkage) Glycosidic bonds between proteins and carbohydrates N -linked oligosaccharides Blood group antigens Blood group antigens • Carbohydrates attached to glycoproteins, glycolipids on the surfaces of RBCs – Antigenic determinants • If an antigen not normally present in a person is introduced, the person's immune system recognizes it as foreign Glycosaminoglycans • have disaccharide repeating units containing a derivative of an amino sugar – either glucosamine or galactosamine • At least one of the sugars in the repeating unit has a negatively charged carboxylate or sulfate group • usually attached to proteins to form proteoglycans The major glycosaminoglycans Proteoglycans • Almost all GAGs covalently attached to protein in the form of proteoglycans • distinguished from other glycoproteins by the nature, quantity, and arrangement of their sugar side chains • Found in animal cells The linkage between a GAG chain and its core protein in a proteoglycan molecule Glycoproteins vs proteoglycans 1. Glycoproteins contain 1–60% carbohydrate by weight numerous relatively short, branched oligosaccharide chains 2. Proteoglycans can contain as much as 95% carbohydrate by weight Most carbs = long, unbranched GAG chains, each typically ~ 80 sugars long Proteoglycan functions • GAGs, proteoglycans can associate to form huge polymeric complexes in the ECM • GAG chains can form gels of varying pore size, charge density – may serve as selective sieves to regulate the traffic of molecules and cells according to their size, charge, or both • Also associates with fibrous matrix proteins, e.g., collagen • Aggrecan – major component of cartilage – MW ~ 3 × 106 daltons with over 100 GAG chains • Decorin – secreted by fibroblasts – has a single GAG chain