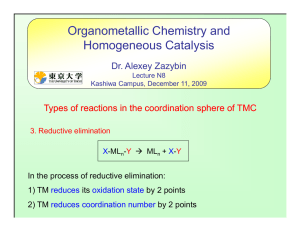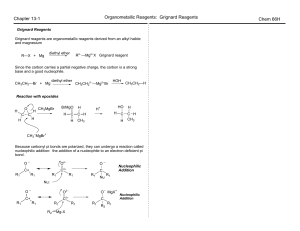
2 - Humble ISD
... 1. burn them - destroying the whole molecule 2. react them with some of the halogens, breaking carbon-hydrogen bonds; 3. crack them, breaking carbon-carbon bonds. ...
... 1. burn them - destroying the whole molecule 2. react them with some of the halogens, breaking carbon-hydrogen bonds; 3. crack them, breaking carbon-carbon bonds. ...
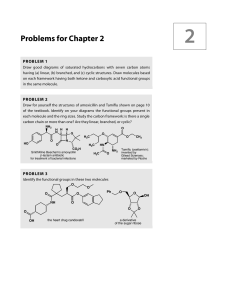
Problems for Chapter 2
... PROB LE M 7 Four compounds having the formula C4H6O2 have the IR and NMR data given below. How many DBEs (double bond equivalents—see p. 75 in the textbook) are there in C4H6O2? What are the structures of the four compounds? You might again find it useful to draw a few structures to start with. (a) ...
... PROB LE M 7 Four compounds having the formula C4H6O2 have the IR and NMR data given below. How many DBEs (double bond equivalents—see p. 75 in the textbook) are there in C4H6O2? What are the structures of the four compounds? You might again find it useful to draw a few structures to start with. (a) ...
REVIEWS Environmental remediation by photocatalysis R. Vinu AND Giridhar Madras
... and mechanism of photocatalysis,8–23 with special emphasis on the electron transfer processes, lattice and electronic structure of TiO2 , surface chemistry of semiconductor oxides, generation of reactive radicals, chemisorption of small and large molecules, surface modification by doping, photooxidat ...
... and mechanism of photocatalysis,8–23 with special emphasis on the electron transfer processes, lattice and electronic structure of TiO2 , surface chemistry of semiconductor oxides, generation of reactive radicals, chemisorption of small and large molecules, surface modification by doping, photooxidat ...
Chapter 6 Addition Reactions to Alkenes
... As we have seen, according the Markovnikov’s rule, the electrophile adds to the less substituted carbon so as to allow the positive charge to be supported at the more substituted and therefore more electron rich carbon. In the present case, the addition of the electrophile (the boron) and the nucleo ...
... As we have seen, according the Markovnikov’s rule, the electrophile adds to the less substituted carbon so as to allow the positive charge to be supported at the more substituted and therefore more electron rich carbon. In the present case, the addition of the electrophile (the boron) and the nucleo ...
4134gdisk doc..4134gdisk chapter .. Page501
... oxime–imine complexes.61 In(I), V(II), Eu(II) and Ti(III) all reduce riboflavin with an initial step in which the riboflavin radical ion is produced.62 The reaction is autocatalytic for the outer-sphere V(II) and Ti(III) reactions leading to formation of dihydroriboflavin. ...
... oxime–imine complexes.61 In(I), V(II), Eu(II) and Ti(III) all reduce riboflavin with an initial step in which the riboflavin radical ion is produced.62 The reaction is autocatalytic for the outer-sphere V(II) and Ti(III) reactions leading to formation of dihydroriboflavin. ...
Fundamental Equilibrium Concepts
... A chemical reaction is usually written in a way that suggests it proceeds in one direction, the direction in which we read, but all chemical reactions are reversible, and both the forward and reverse reaction occur to one degree or another depending on conditions. In a chemical equilibrium, the forw ...
... A chemical reaction is usually written in a way that suggests it proceeds in one direction, the direction in which we read, but all chemical reactions are reversible, and both the forward and reverse reaction occur to one degree or another depending on conditions. In a chemical equilibrium, the forw ...
Sample
... The simultaneous addition of boron and hydrogen to the double bond leads to a syn addition: Boron and hydrogen add across the double bond on the same side of the molecule. (If they added to opposite sides of the molecule, the process would be an anti addition.) The stereochemistry of the hydroborati ...
... The simultaneous addition of boron and hydrogen to the double bond leads to a syn addition: Boron and hydrogen add across the double bond on the same side of the molecule. (If they added to opposite sides of the molecule, the process would be an anti addition.) The stereochemistry of the hydroborati ...
Solutions - ChemConnections
... Magnesium is an alkaline earth metal; Mg will oxidize to Mg2+. The oxidation state of hydrogen in HCl is +1. To be reduced, the oxidation state of H must decrease. The obvious choice for the hydrogen product is H2(g), where hydrogen has a zero oxidation state. The balanced reaction is Mg(s) + 2HCl(a ...
... Magnesium is an alkaline earth metal; Mg will oxidize to Mg2+. The oxidation state of hydrogen in HCl is +1. To be reduced, the oxidation state of H must decrease. The obvious choice for the hydrogen product is H2(g), where hydrogen has a zero oxidation state. The balanced reaction is Mg(s) + 2HCl(a ...
Section 1.3 - The Student Room
... Contraction of ion size as the number of protons relative to the number of electrons increases. P3– is the biggest and Cl– is the smallest because of the increasing attraction on the same number of electrons of an increasing number of protons in the nucleus. They each contain an extra shell of elect ...
... Contraction of ion size as the number of protons relative to the number of electrons increases. P3– is the biggest and Cl– is the smallest because of the increasing attraction on the same number of electrons of an increasing number of protons in the nucleus. They each contain an extra shell of elect ...
an introduction to organic reactions
... important studying tools for preparing for tests because they ingrain the material in your head, but more importantly, they help you think about the chemistry in ways that are very useful when taking the midterms and final exam. ...
... important studying tools for preparing for tests because they ingrain the material in your head, but more importantly, they help you think about the chemistry in ways that are very useful when taking the midterms and final exam. ...
Microporous polymer beads for chemical
... Technology. The thesis was assigned by Tor Erik Kristensen and was carried out as collaboration between the group for Energetic Materials and Rocket Motor Propulsion Technology and the group for Chemical Warfare Agents. The latter group deals with issues concerning protection and preparedness agains ...
... Technology. The thesis was assigned by Tor Erik Kristensen and was carried out as collaboration between the group for Energetic Materials and Rocket Motor Propulsion Technology and the group for Chemical Warfare Agents. The latter group deals with issues concerning protection and preparedness agains ...
M.Sc. Chemistry : Syllabus (CBCS)
... To learn the concepts of stereochemistry, conformational analysis and their Application in the determination of reaction mechanism. To understand the mechanism of nucleophilic and electrophilic substitution reactions. UNIT-I: STEREOCHEMISTRY Optical activity and chirality, Classification ofchiral mo ...
... To learn the concepts of stereochemistry, conformational analysis and their Application in the determination of reaction mechanism. To understand the mechanism of nucleophilic and electrophilic substitution reactions. UNIT-I: STEREOCHEMISTRY Optical activity and chirality, Classification ofchiral mo ...
Week 3 July 22, 2016 Worksheet Review III 1 mol = 6.022 × 1023 1
... NiCl2 (aq) + Na2S (aq) → These are both soluble so they will exist as ions in solution: Ni2+ (aq) + Cl– (aq) + Na+ (aq) + S2– (aq) → Now you want to determine the products of this reaction. You can either set up a chart with the cations/anions or swap the two ions. Use the solubility rules to determ ...
... NiCl2 (aq) + Na2S (aq) → These are both soluble so they will exist as ions in solution: Ni2+ (aq) + Cl– (aq) + Na+ (aq) + S2– (aq) → Now you want to determine the products of this reaction. You can either set up a chart with the cations/anions or swap the two ions. Use the solubility rules to determ ...
Alcohols, Ethers, and Epoxides
... alkene (Chapter 12). To name an epoxide this way, mentally replace the epoxide oxygen by a double bond, name the alkene (Section 10.3), and then add the word oxide. For example, the common name for oxirane is ethylene oxide, since it is an epoxide derived from the alkene ethylene. We will use this m ...
... alkene (Chapter 12). To name an epoxide this way, mentally replace the epoxide oxygen by a double bond, name the alkene (Section 10.3), and then add the word oxide. For example, the common name for oxirane is ethylene oxide, since it is an epoxide derived from the alkene ethylene. We will use this m ...
Elimination Reactions
... neutral conditions with polar solvents, such as water, ethyl alcohol or acetic acid. • E1 reactions can also occur with strong bases, but only at low concentration, about 0.01 to 0.1 M or below. • E2 reactions require strong base in high concentration, about 1 M or above. ...
... neutral conditions with polar solvents, such as water, ethyl alcohol or acetic acid. • E1 reactions can also occur with strong bases, but only at low concentration, about 0.01 to 0.1 M or below. • E2 reactions require strong base in high concentration, about 1 M or above. ...
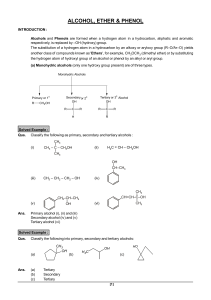
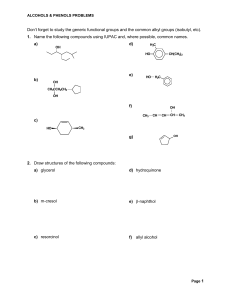
Don`t forget to study the generic functional groups and the common
... ALCOHOLS & PHENOLS PROBLEMS ...
... ALCOHOLS & PHENOLS PROBLEMS ...
Excited State Reactions of Carbonyl Compounds
... (alcohols, hydrocarbons, amines etc.) commonly results in the photochemical process of hydrogen abstraction, followed by product formation via secondary thermal reactions (Scheme 9.1). The primary bond-forming step in this transformation is the addition of a hydrogen atom to the C=O chromophore to f ...
... (alcohols, hydrocarbons, amines etc.) commonly results in the photochemical process of hydrogen abstraction, followed by product formation via secondary thermal reactions (Scheme 9.1). The primary bond-forming step in this transformation is the addition of a hydrogen atom to the C=O chromophore to f ...
Experiments in General Chemistry: Featuring MeasureNet
... Chemists observe matter by determining, measuring, and monitoring physical and chemical properties of matter. A property is any characteristic that can be used to describe matter (e.g., size, color, mass, density, solubility, etc.). In this experiment, we will determine the density of liquids and so ...
... Chemists observe matter by determining, measuring, and monitoring physical and chemical properties of matter. A property is any characteristic that can be used to describe matter (e.g., size, color, mass, density, solubility, etc.). In this experiment, we will determine the density of liquids and so ...
Strychnine total synthesis

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field. At the time it formed the natural conclusion to an elaborate process of molecular structure elucidation that started with the isolation of strychnine from the beans of Strychnos ignatii by Pierre Joseph Pelletier and Joseph Bienaimé Caventou in 1818. Major contributors to the entire effort were Sir Robert Robinson with over 250 publications and Hermann Leuchs with another 125 papers in a time span of 40 years. Robinson was awarded the Nobel Prize in Chemistry in 1947 for his work on alkaloids, strychnine included. The process of chemical identification was completed with publications in 1946 by Robinson and later confirmed by Woodward in 1947. X-ray structures establishing the absolute configuration became available between 1947 and 1951 with publications from J. M. Bijvoet and J.H. Robertson .Woodward published a very brief account on the strychnine synthesis in 1954 (just 3 pages) and a lengthy one (42 pages) in 1963.Many more methods exist and reported by the research groups of Magnus, Overman, Kuehne, Rawal, Bosch, Vollhardt, Mori, Shibasaki, Li, Fukuyama Vanderwal and MacMillan. Synthetic (+)-strychnine is also known. Racemic synthesises were published by Padwa in 2007 and in 2010 by Andrade and by Reissig.In his 1963 publication Woodward quoted Sir Robert Robinson who said for its molecular size it is the most complex substance known.