Chemical Equations
... combine sodium and carbonate into a neutral compound is to use two sodiums for each carbonate. Note how the ammonium carbonate required parentheses, as two ammoniums were needed to balance the charge of the carbonate. Subscripts are used within a chemical formula to show the number of atoms in a sin ...
... combine sodium and carbonate into a neutral compound is to use two sodiums for each carbonate. Note how the ammonium carbonate required parentheses, as two ammoniums were needed to balance the charge of the carbonate. Subscripts are used within a chemical formula to show the number of atoms in a sin ...
rate of chemical reaction and chemical equilibrium
... chemical reaction but there is no change in the catalyst itself. In the presence of a catalyst a reaction takes place at a faster rate and at lower temperature. Iron is used as a catalyst in the manufacture of ammonia. Iron catalyst increases the rate of reaction between nitrogen and hydrogen to for ...
... chemical reaction but there is no change in the catalyst itself. In the presence of a catalyst a reaction takes place at a faster rate and at lower temperature. Iron is used as a catalyst in the manufacture of ammonia. Iron catalyst increases the rate of reaction between nitrogen and hydrogen to for ...
AP Chemistry Summer Assignment
... You need to memorize the following solubility rules for determining your ppt (precipitate). 1. Common sodium ions, potassium ions, ammonium ions, and hydrogen ion compounds are soluble in water. (If soluble in water they will not precipitate out of the chemical environment.) 2. Common nitr ...
... You need to memorize the following solubility rules for determining your ppt (precipitate). 1. Common sodium ions, potassium ions, ammonium ions, and hydrogen ion compounds are soluble in water. (If soluble in water they will not precipitate out of the chemical environment.) 2. Common nitr ...
Reactions of Carbonyl compounds
... CN¯ acts as a nucleophile and attacks the slightly positive C One of the C=O bonds breaks; a pair of electrons goes onto the O ...
... CN¯ acts as a nucleophile and attacks the slightly positive C One of the C=O bonds breaks; a pair of electrons goes onto the O ...
Unit 2 Chemical Reactions
... a. Calcium carbide ( CaC2 ) reacts with water to form acetylene. Collect a test tube of acetylene as follows: - Half fill a beaker with water. - Invert a test tube full of water into the beaker. - Use forceps to drop a small piece of calcium carbide into the water. - Place the inverted test tube ove ...
... a. Calcium carbide ( CaC2 ) reacts with water to form acetylene. Collect a test tube of acetylene as follows: - Half fill a beaker with water. - Invert a test tube full of water into the beaker. - Use forceps to drop a small piece of calcium carbide into the water. - Place the inverted test tube ove ...
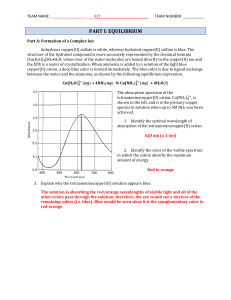
Equilibrium
... Often reactions are written with only ions that are actually involved in the reaction. This is why the nitrate and potassium ions have been left off of the equation. These ions that are left off the equation are called spectator ions. Write this equation and below each chemical list the solution col ...
... Often reactions are written with only ions that are actually involved in the reaction. This is why the nitrate and potassium ions have been left off of the equation. These ions that are left off the equation are called spectator ions. Write this equation and below each chemical list the solution col ...
T10 SL - MsReenChemistry
... Two compounds, A and D, each have the formula C4H9Cl. Compound A is reacted with dilute aqueous sodium hydroxide to produce compound B with a formula of C4H10O. Compound B is then oxidized with acidified potassium manganate(VII) to produce compound C with a formula of C4H8O. Compound C resists furth ...
... Two compounds, A and D, each have the formula C4H9Cl. Compound A is reacted with dilute aqueous sodium hydroxide to produce compound B with a formula of C4H10O. Compound B is then oxidized with acidified potassium manganate(VII) to produce compound C with a formula of C4H8O. Compound C resists furth ...
PREPARATION OF ORGANOLITHIUM COMPOUNDS - GCG-42
... by following methods:By Halogen Metal Exchange From Terminal Alkynes By Trans Metalation/ Metal-Metal Exchange By Directed Metalation/ Ortho Metalation ...
... by following methods:By Halogen Metal Exchange From Terminal Alkynes By Trans Metalation/ Metal-Metal Exchange By Directed Metalation/ Ortho Metalation ...
Unit 2: Carbon Compounds
... Unsaturated hydrocarbons contain at least one carbon to carbon double covalent bond. ...
... Unsaturated hydrocarbons contain at least one carbon to carbon double covalent bond. ...
Chapter 16
... The reaction involves an elimination reaction that gives a triple bond The intermediate is called benzyne ...
... The reaction involves an elimination reaction that gives a triple bond The intermediate is called benzyne ...
Unit 2: Carbon Compounds
... Unsaturated hydrocarbons contain at least one carbon to carbon double covalent bond. ...
... Unsaturated hydrocarbons contain at least one carbon to carbon double covalent bond. ...
Suggest a reason for the large difference in the boiling points of
... manufactured by Deacons process . identify this gas . and write reactions of this gas with NaoH. 6 Why is helium used in diving apparatus? 7 Why has it been difficult to study the chemistry of radon? 8 Draw structure of 1) H2S2O7 2)HClO3 3) IF7 4) XeO3 9 Describe manufacturing of H2SO4 by contact pr ...
... manufactured by Deacons process . identify this gas . and write reactions of this gas with NaoH. 6 Why is helium used in diving apparatus? 7 Why has it been difficult to study the chemistry of radon? 8 Draw structure of 1) H2S2O7 2)HClO3 3) IF7 4) XeO3 9 Describe manufacturing of H2SO4 by contact pr ...
Unit 4 - cloudfront.net
... 3. Substances with the nonmetal in its maximum ox. number = oxidizing agent. (NO3- = ______). 4. Substances with the nonmetal in its minimum ox. number = reducing agent. (H2S = ______). 5. Substances with an intermediate oxidation number = oxidizing or reducing agent. ...
... 3. Substances with the nonmetal in its maximum ox. number = oxidizing agent. (NO3- = ______). 4. Substances with the nonmetal in its minimum ox. number = reducing agent. (H2S = ______). 5. Substances with an intermediate oxidation number = oxidizing or reducing agent. ...
Answers - Scioly.org
... The peak of highest mass to charge ratio is approximately 116; therefore, the unknown molecule would have a molecular mass of 116. Ethyl butanoate has the chemical formula C6H12O2, which would have the same molecular mass seen from the mass spectrum and the same empirical formula shown in the combus ...
... The peak of highest mass to charge ratio is approximately 116; therefore, the unknown molecule would have a molecular mass of 116. Ethyl butanoate has the chemical formula C6H12O2, which would have the same molecular mass seen from the mass spectrum and the same empirical formula shown in the combus ...
COUNTING ATOMS
... number of each atom present. B. A representation of a chemical reaction expressed as a formula. C. Substances that change in a reaction. D. The new substances that are formed as a result of the reaction. ...
... number of each atom present. B. A representation of a chemical reaction expressed as a formula. C. Substances that change in a reaction. D. The new substances that are formed as a result of the reaction. ...
Chapter 07
... • Up to 4 carbons, acid is miscible in water. • More soluble in alcohol. • Also soluble in relatively nonpolar solvents like chloroform because it dissolves as a dimer. ...
... • Up to 4 carbons, acid is miscible in water. • More soluble in alcohol. • Also soluble in relatively nonpolar solvents like chloroform because it dissolves as a dimer. ...
Lipids
... Sphingolipids are amino alcohols – note the amide in R2 and vinyl group in R3 • Named in the 1920s, when Egyptology was all the rage (“Sphinx” = mysterious) and these compounds were discovered and their function not known at the time • Now known to have many functions, including nerve cell sheaths ...
... Sphingolipids are amino alcohols – note the amide in R2 and vinyl group in R3 • Named in the 1920s, when Egyptology was all the rage (“Sphinx” = mysterious) and these compounds were discovered and their function not known at the time • Now known to have many functions, including nerve cell sheaths ...
organic outline - No Brain Too Small
... CH3CH3 + 3½O2 2CO2 + 3H2O • Plentiful O2 needed for complete combustion; clean flame Or 2CH3CH3 + 7O2 4CO2 + 6H2O Products H2O + CO2 + maximum energy released Hint. Balance in order C H O….. • Limited O2 get incomplete combustion; yellow and sooty flame Products H2O + C + CO + less energy released ...
... CH3CH3 + 3½O2 2CO2 + 3H2O • Plentiful O2 needed for complete combustion; clean flame Or 2CH3CH3 + 7O2 4CO2 + 6H2O Products H2O + CO2 + maximum energy released Hint. Balance in order C H O….. • Limited O2 get incomplete combustion; yellow and sooty flame Products H2O + C + CO + less energy released ...
Porous silicon-based nanostructured microparticles as degradable
... Synthesis of 11-undecenylamine (Schematic 1 (2)): LiAlH4 (2.7 g, 71.1 mmol) was placed in a 250 mL round-bottom flask containing 50 mL of anhydrous THF, and the mixture was heated at reflux for 30 minutes under N2. Heating was stopped and a solution of 9-undecenamide (5.0 g, 27.3 mmol) in 100 mL of ...
... Synthesis of 11-undecenylamine (Schematic 1 (2)): LiAlH4 (2.7 g, 71.1 mmol) was placed in a 250 mL round-bottom flask containing 50 mL of anhydrous THF, and the mixture was heated at reflux for 30 minutes under N2. Heating was stopped and a solution of 9-undecenamide (5.0 g, 27.3 mmol) in 100 mL of ...
Strychnine total synthesis

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field. At the time it formed the natural conclusion to an elaborate process of molecular structure elucidation that started with the isolation of strychnine from the beans of Strychnos ignatii by Pierre Joseph Pelletier and Joseph Bienaimé Caventou in 1818. Major contributors to the entire effort were Sir Robert Robinson with over 250 publications and Hermann Leuchs with another 125 papers in a time span of 40 years. Robinson was awarded the Nobel Prize in Chemistry in 1947 for his work on alkaloids, strychnine included. The process of chemical identification was completed with publications in 1946 by Robinson and later confirmed by Woodward in 1947. X-ray structures establishing the absolute configuration became available between 1947 and 1951 with publications from J. M. Bijvoet and J.H. Robertson .Woodward published a very brief account on the strychnine synthesis in 1954 (just 3 pages) and a lengthy one (42 pages) in 1963.Many more methods exist and reported by the research groups of Magnus, Overman, Kuehne, Rawal, Bosch, Vollhardt, Mori, Shibasaki, Li, Fukuyama Vanderwal and MacMillan. Synthetic (+)-strychnine is also known. Racemic synthesises were published by Padwa in 2007 and in 2010 by Andrade and by Reissig.In his 1963 publication Woodward quoted Sir Robert Robinson who said for its molecular size it is the most complex substance known.