TYPES OF CHEMICAL REACTIONS AND SOLUTION CHEMISTRY
... 7. The _____ and the _____ cannot coexist in solution because water is a nonelectrolyte. ...
... 7. The _____ and the _____ cannot coexist in solution because water is a nonelectrolyte. ...
Chapter 3 Part 2 Review
... Titanium is a strong, lightweight, corrosionresistant metal that is used in rockets, aircraft, jet engines and bicycle frames. It is prepared by the reaction of titanium (IV) chloride with molten magnesium between 950oC and 1150oC: TiCl4(g) + 2Mg(l) Ti(s) + 2MgCl2(l) 3.54x107 g of TiCl4 are reac ...
... Titanium is a strong, lightweight, corrosionresistant metal that is used in rockets, aircraft, jet engines and bicycle frames. It is prepared by the reaction of titanium (IV) chloride with molten magnesium between 950oC and 1150oC: TiCl4(g) + 2Mg(l) Ti(s) + 2MgCl2(l) 3.54x107 g of TiCl4 are reac ...
3.0 Hess`s Law
... • Because these two reactions occur simultaneously, it is not possible to directly measure the enthalpy of formation of CO(g) from C(s) and O2(g). • We do know the enthalpy of formation of carbon dioxide and the enthalpy of combustion of carbon monoxide: ...
... • Because these two reactions occur simultaneously, it is not possible to directly measure the enthalpy of formation of CO(g) from C(s) and O2(g). • We do know the enthalpy of formation of carbon dioxide and the enthalpy of combustion of carbon monoxide: ...
Enantiodivergent conversion of chiral secondary alcohols into
... •Only works for aryl alcohols •Aryl boranes (e.g. Ph-9-BBN) incompatible due to protodeboronation during aqueous oxidative work-up •Indanol-derived carbamate gives same enantiomer (retention) with triethyl borane or ethylboronic acid (pyramidalization of geometrically constrained carbanion) ...
... •Only works for aryl alcohols •Aryl boranes (e.g. Ph-9-BBN) incompatible due to protodeboronation during aqueous oxidative work-up •Indanol-derived carbamate gives same enantiomer (retention) with triethyl borane or ethylboronic acid (pyramidalization of geometrically constrained carbanion) ...
Acids-bases and Organic Review
... 35. A straight-chain hydrocarbon that has only one double bond in each molecule has the general formula 1. CnH2n-6 3. CnH2n 2. CnH2n-2 ...
... 35. A straight-chain hydrocarbon that has only one double bond in each molecule has the general formula 1. CnH2n-6 3. CnH2n 2. CnH2n-2 ...
Advanced Organic chemistry syllabus
... 6- Shows clearly full consciousness of ethics in all aspects of scientific research including novel drug in vivo testing. B) Intellectual Skills: Upon completion of the course, students will be able to: 1- Proper handling of chemical reagents and the assignment of safety sheet. 1-Design possible syn ...
... 6- Shows clearly full consciousness of ethics in all aspects of scientific research including novel drug in vivo testing. B) Intellectual Skills: Upon completion of the course, students will be able to: 1- Proper handling of chemical reagents and the assignment of safety sheet. 1-Design possible syn ...
Enantioselective Organocatalytic Aminomethylation of Aldehydes: A
... reactants. The Mannich reaction products, R-substituted β-amino aldehydes, were immediately reduced to the corresponding β-substituted γ-amino alcohols to avoid epimerization. Initial studies involving pentanal revealed modest enantioselectivity when the reaction was carried out with 20 mol % cataly ...
... reactants. The Mannich reaction products, R-substituted β-amino aldehydes, were immediately reduced to the corresponding β-substituted γ-amino alcohols to avoid epimerization. Initial studies involving pentanal revealed modest enantioselectivity when the reaction was carried out with 20 mol % cataly ...
Organic Chemistry I (CHEM 2010 and 2012)
... expected to read and study the material to be discussed prior to the lecture. This includes working in-chapter and end-of-chapter problems and exercises in the text. Students should review the material discussed until comprehension is acquired and seek assistance when necessary. It is also highly re ...
... expected to read and study the material to be discussed prior to the lecture. This includes working in-chapter and end-of-chapter problems and exercises in the text. Students should review the material discussed until comprehension is acquired and seek assistance when necessary. It is also highly re ...
Chem 1A Practice Final
... 7. A solution is prepared by dissolving 0.115 moles of ammonium sulfate, (NH4)2SO4, in enough water to make 100.0 mL of stock solution. A 11.00 mL sample of this stock solution is added to 50.00 mL of water. Calculate the concentration of ammonium ions in the final solution. a) 1.15 M b) 0.51 M c) ...
... 7. A solution is prepared by dissolving 0.115 moles of ammonium sulfate, (NH4)2SO4, in enough water to make 100.0 mL of stock solution. A 11.00 mL sample of this stock solution is added to 50.00 mL of water. Calculate the concentration of ammonium ions in the final solution. a) 1.15 M b) 0.51 M c) ...
4.14 Halogenation of Alkanes RH + X2 → RX + HX RH + X2 → RX +
... substitution of tertiary hydrogens. Major synthetic application is in synthesis of tertiary alkyl bromides. ...
... substitution of tertiary hydrogens. Major synthetic application is in synthesis of tertiary alkyl bromides. ...
Equilibrium (Sheet 1)
... is said to shift to the right, which means that the relative concentration of the reactant will decrease and the relative concentration of the products will increase. It can be reasoned that if the concentration of CO is increased, then the equilibrium will shift to the right, while increasing the c ...
... is said to shift to the right, which means that the relative concentration of the reactant will decrease and the relative concentration of the products will increase. It can be reasoned that if the concentration of CO is increased, then the equilibrium will shift to the right, while increasing the c ...
Chemistry Spell check on
... 5 If this information is correct, print your name and seat number in the boxes provided. 6 The answer to each question is either A, B, C or D. Decide what your answer is, then, using your pencil, put a horizontal line in the space provided (see sample question below). 7 There is only one co ...
... 5 If this information is correct, print your name and seat number in the boxes provided. 6 The answer to each question is either A, B, C or D. Decide what your answer is, then, using your pencil, put a horizontal line in the space provided (see sample question below). 7 There is only one co ...
Silica supported zinc chloride catalyzed acetylation of amines
... with silica gel. Up to 310ºC, there is no possibility of the loss of physisorbed and chemisorbed ZnCl2 from the surface of silica gel. Therefore, it is safe to carry out the reaction at 80ºC. For optimizing the reaction conditions, the primary emphasis is laid on the selection of solvent for carryin ...
... with silica gel. Up to 310ºC, there is no possibility of the loss of physisorbed and chemisorbed ZnCl2 from the surface of silica gel. Therefore, it is safe to carry out the reaction at 80ºC. For optimizing the reaction conditions, the primary emphasis is laid on the selection of solvent for carryin ...
Organic Compounds
... between functional groups provide reasons for differences in chemical reactivity. In preparation for this laboratory exercise, review the sections in your text book pertaining to molecular structure of compounds such as alkanes, alkenes, alkynes, aromatics, alcohols, ethers, aldehydes, ketones, carb ...
... between functional groups provide reasons for differences in chemical reactivity. In preparation for this laboratory exercise, review the sections in your text book pertaining to molecular structure of compounds such as alkanes, alkenes, alkynes, aromatics, alcohols, ethers, aldehydes, ketones, carb ...
GENERAL CHEMISTRY REVIEW
... Cl- (-1), Fe2+ (+2), Fe3+ (+3), S2- (-2), Ca2+ (+2), H+ (+1) etc 3. Certain elements when present in compounds have common oxidation states. a) alkali metals (Li+, Na+, K+) are always +1 b) alkali earth metals (Mg2+, Ca2+, Sr2+, Ba2+) are always +2 c) hydrogen is +1 (except in metal hydride compound ...
... Cl- (-1), Fe2+ (+2), Fe3+ (+3), S2- (-2), Ca2+ (+2), H+ (+1) etc 3. Certain elements when present in compounds have common oxidation states. a) alkali metals (Li+, Na+, K+) are always +1 b) alkali earth metals (Mg2+, Ca2+, Sr2+, Ba2+) are always +2 c) hydrogen is +1 (except in metal hydride compound ...
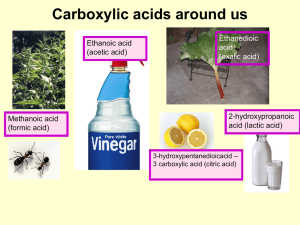
Acidic and Basic Character of Carboxylic Acids
... A second method for preparing a carboxylic acid with an additional carbon is through the synthesis and hydrolysis of a nitrile, RCN. ...
... A second method for preparing a carboxylic acid with an additional carbon is through the synthesis and hydrolysis of a nitrile, RCN. ...
Direct production of hydrogen peroxide from CO, O2, and H2O over
... copper catalyst exhibits superior performance with respect to other metals for H2O2 synthesis, as it is known to be one of the most efficient systems for the WGS reaction.14 On the other hand, it was found that the catalytic activity of Cu/Al2O3 is greatly dependent on a number of important factors su ...
... copper catalyst exhibits superior performance with respect to other metals for H2O2 synthesis, as it is known to be one of the most efficient systems for the WGS reaction.14 On the other hand, it was found that the catalytic activity of Cu/Al2O3 is greatly dependent on a number of important factors su ...
AP Chemistry Summer Assignment
... 63. A 2.0g sample of SX6 (g) has a volume of 329.5 cm3 at 1.00 atm and 20oC. Identify the element ‘X’. Name the compound. 64. When Hydrogen sulfide gas, H2S, reacts with oxygen, Sulfur dioxide gas and steam are produced. a. Write the balanced chemical equation for this reaction. b. How many liters o ...
... 63. A 2.0g sample of SX6 (g) has a volume of 329.5 cm3 at 1.00 atm and 20oC. Identify the element ‘X’. Name the compound. 64. When Hydrogen sulfide gas, H2S, reacts with oxygen, Sulfur dioxide gas and steam are produced. a. Write the balanced chemical equation for this reaction. b. How many liters o ...
Hydrocarbons
... oxidised to create carboxylic acids. We can use acidified dichromate or acidified permanganate as oxidising agents Elimination – As you might have guessed elimination reactions remove parts of the alcohol. In this case we remove the –OH group and one other hydrogen using concentrated sulfuric acid. ...
... oxidised to create carboxylic acids. We can use acidified dichromate or acidified permanganate as oxidising agents Elimination – As you might have guessed elimination reactions remove parts of the alcohol. In this case we remove the –OH group and one other hydrogen using concentrated sulfuric acid. ...
in a Chemical Reactor - Max-Planck
... when the researchers from Magdeburg produce cyclohexanol out of cyclohexene using formic acid cyclohexyl esters. ...
... when the researchers from Magdeburg produce cyclohexanol out of cyclohexene using formic acid cyclohexyl esters. ...
synthesis, chemistry and optical resol
... arrangement of the two rings was found to block additions to the buried double bond, resulting in dramatic reactivity differences between the trans (13, n = 12) and cis (12, n = 12) i~omers.l-~ We now wish to record the synthesis of [20.10]-, [22.10]-, and [26.10]betweenanenes (13a-c) by a new route ...
... arrangement of the two rings was found to block additions to the buried double bond, resulting in dramatic reactivity differences between the trans (13, n = 12) and cis (12, n = 12) i~omers.l-~ We now wish to record the synthesis of [20.10]-, [22.10]-, and [26.10]betweenanenes (13a-c) by a new route ...
2012 C13 Exam answers
... 32 Which statement about catalysts is incorrect? 28 A cylinder of unknown volume contains helium gas, He(g), at 3.50 atm and 315 K. The helium gas is then transferred to a 7.0 L gas cylinder containing Ne(g), at 2.50 atm and 315 K. If the final total pressure at 315 K is 5.75 atm, then what is the v ...
... 32 Which statement about catalysts is incorrect? 28 A cylinder of unknown volume contains helium gas, He(g), at 3.50 atm and 315 K. The helium gas is then transferred to a 7.0 L gas cylinder containing Ne(g), at 2.50 atm and 315 K. If the final total pressure at 315 K is 5.75 atm, then what is the v ...
Strychnine total synthesis

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field. At the time it formed the natural conclusion to an elaborate process of molecular structure elucidation that started with the isolation of strychnine from the beans of Strychnos ignatii by Pierre Joseph Pelletier and Joseph Bienaimé Caventou in 1818. Major contributors to the entire effort were Sir Robert Robinson with over 250 publications and Hermann Leuchs with another 125 papers in a time span of 40 years. Robinson was awarded the Nobel Prize in Chemistry in 1947 for his work on alkaloids, strychnine included. The process of chemical identification was completed with publications in 1946 by Robinson and later confirmed by Woodward in 1947. X-ray structures establishing the absolute configuration became available between 1947 and 1951 with publications from J. M. Bijvoet and J.H. Robertson .Woodward published a very brief account on the strychnine synthesis in 1954 (just 3 pages) and a lengthy one (42 pages) in 1963.Many more methods exist and reported by the research groups of Magnus, Overman, Kuehne, Rawal, Bosch, Vollhardt, Mori, Shibasaki, Li, Fukuyama Vanderwal and MacMillan. Synthetic (+)-strychnine is also known. Racemic synthesises were published by Padwa in 2007 and in 2010 by Andrade and by Reissig.In his 1963 publication Woodward quoted Sir Robert Robinson who said for its molecular size it is the most complex substance known.