3 - Study Hungary
... A: the atomic number decreases by 2 and the mass number by 4. B: the atomic number decreases by 4 and the mass number by 2. C: the atomic number increases by 1 and the mass number doesn’t change. D: the loss of a neutron decreases the mass number by 1 and the charge by 1. E: the loss of a proton dec ...
... A: the atomic number decreases by 2 and the mass number by 4. B: the atomic number decreases by 4 and the mass number by 2. C: the atomic number increases by 1 and the mass number doesn’t change. D: the loss of a neutron decreases the mass number by 1 and the charge by 1. E: the loss of a proton dec ...
today`s PowerPoint
... • You can use 2,4 – dinitrophenylhydrazine or 2,4-DNP to test for the carbonyl group. • A solution of 2,4-DNP, methanol and H2SO4 is known as Brady’s reagent. • A positive test will give an orange/yellow precipitate. • Both aldehydes and ketones will test positively. No other compounds (e.g. Carboxy ...
... • You can use 2,4 – dinitrophenylhydrazine or 2,4-DNP to test for the carbonyl group. • A solution of 2,4-DNP, methanol and H2SO4 is known as Brady’s reagent. • A positive test will give an orange/yellow precipitate. • Both aldehydes and ketones will test positively. No other compounds (e.g. Carboxy ...
Copper(II) bromide as efficient catalyst for silyl
... The reaction rates were thus mostly dependent on the size of the substituents at the core Si atom. Interestingly, they approximately followed the rate order of acid-catalyzed hydrolysis of silyl ethers, known to be due to increasing substituent size and electronic effects around the Si atom. 2 It is ...
... The reaction rates were thus mostly dependent on the size of the substituents at the core Si atom. Interestingly, they approximately followed the rate order of acid-catalyzed hydrolysis of silyl ethers, known to be due to increasing substituent size and electronic effects around the Si atom. 2 It is ...
Aspirin - Community Colleges of Spokane
... Aspirin, acetylsalicylic acid, has been used as an antipyretic (fever reducer), an analgesic (pain reliever), an antirheumatic, and recently as an anticoagulant and anti-inflammatory drug. Aspirin is truly a wonder drug, an achievement to modern medicine. In this experiment, you will synthesize acet ...
... Aspirin, acetylsalicylic acid, has been used as an antipyretic (fever reducer), an analgesic (pain reliever), an antirheumatic, and recently as an anticoagulant and anti-inflammatory drug. Aspirin is truly a wonder drug, an achievement to modern medicine. In this experiment, you will synthesize acet ...
Document
... 8. A weighed sample of crystalline sodium carbonate Na2 CO3 and H2O, was heated in a crucible until there was no further change in mass. The mass of the sample reduced by 145%. Calculate the number of moles (n) of the water of crystallization ( Na = 23, O=16, C = 12 H = 1 ) ( 3 Marks ) ...
... 8. A weighed sample of crystalline sodium carbonate Na2 CO3 and H2O, was heated in a crucible until there was no further change in mass. The mass of the sample reduced by 145%. Calculate the number of moles (n) of the water of crystallization ( Na = 23, O=16, C = 12 H = 1 ) ( 3 Marks ) ...
Bond angles - Nayland College
... To 5 mL of Benedict’s solution in a tt, add 5 drops of the test substance. Test ethanol, ethanal and propanone. Heat to boiling CAREFULLY over a Bunsen Burner. ...
... To 5 mL of Benedict’s solution in a tt, add 5 drops of the test substance. Test ethanol, ethanal and propanone. Heat to boiling CAREFULLY over a Bunsen Burner. ...
organic chemistry
... • The longest continuous chain must contain the double bond. • The base name now ends in –ene. • The carbons are numbered so as to keep the number for the double bond as low as possible. • The base name is given a number which identifies the location of the double bond. – An alkyne is a hydrocarbon ...
... • The longest continuous chain must contain the double bond. • The base name now ends in –ene. • The carbons are numbered so as to keep the number for the double bond as low as possible. • The base name is given a number which identifies the location of the double bond. – An alkyne is a hydrocarbon ...
Ch06 BalancingChemRxns
... Solid iron rusts. Solid iron reacts with oxygen gas to produce iron(III) oxide. ...
... Solid iron rusts. Solid iron reacts with oxygen gas to produce iron(III) oxide. ...
CHM230 - Preparation of Methyl Benzoate Preparation of Methyl
... position of the equilibrium can be shifted by adding more of the acid or of the alcohol, depending on cost or availability. The mechanism of the reaction involves initial protonation of the carboxyl group, attack by the nucleophilic hydroxyl, a proton transfer, and loss of water followed by loss of ...
... position of the equilibrium can be shifted by adding more of the acid or of the alcohol, depending on cost or availability. The mechanism of the reaction involves initial protonation of the carboxyl group, attack by the nucleophilic hydroxyl, a proton transfer, and loss of water followed by loss of ...
Geoffrey Wilkinson - Nobel Lecture
... Indeed, the activity of many metal complexes with π-bonding ligands present in catalytic reactions depends on the lability of the metal of carbon bonds. Despite all the intense study of metal-carbon bonds and the arguments about their stability, it is remarkable that only few bond energies are known ...
... Indeed, the activity of many metal complexes with π-bonding ligands present in catalytic reactions depends on the lability of the metal of carbon bonds. Despite all the intense study of metal-carbon bonds and the arguments about their stability, it is remarkable that only few bond energies are known ...
functional groups - Solon City Schools
... UNSATURATED: ALKENES AND ALKYNES • alkenes: contain at least one double bond • sp2 hybridized • Trigonal planar • CnH2n ...
... UNSATURATED: ALKENES AND ALKYNES • alkenes: contain at least one double bond • sp2 hybridized • Trigonal planar • CnH2n ...
Module_16_-_Industrial_and_organic_chemistry
... Haematite is the ore which contains lots of the compound iron oxide. To extract iron from the compound, it is heated in a furnace with carbon. The carbon removes the oxygen from the iron oxide to leave just iron. The removal of oxygen from a compound is called REDUCTION. [The gain of oxygen is calle ...
... Haematite is the ore which contains lots of the compound iron oxide. To extract iron from the compound, it is heated in a furnace with carbon. The carbon removes the oxygen from the iron oxide to leave just iron. The removal of oxygen from a compound is called REDUCTION. [The gain of oxygen is calle ...
Flexi-Biogas - Pwani University
... until a point of degradation occurs. Being that organisms are responsible for the digestion process, it is vital to ensure that the entire process is kept within a certain temperature range to maximize reaction speed and organism livelihood. That being said, the range depends on the species of organ ...
... until a point of degradation occurs. Being that organisms are responsible for the digestion process, it is vital to ensure that the entire process is kept within a certain temperature range to maximize reaction speed and organism livelihood. That being said, the range depends on the species of organ ...
Chemistry - SchoolNotes.com
... 24) What is a limiting reagent? A reactant with a specific amount that limits the amount of th product produced. 25) How is a limiting reactant problem different from other stoichiometry problems? (What is your clue that the problem involves a limiting reactant?) 26) Lithium nitride is prepared by t ...
... 24) What is a limiting reagent? A reactant with a specific amount that limits the amount of th product produced. 25) How is a limiting reactant problem different from other stoichiometry problems? (What is your clue that the problem involves a limiting reactant?) 26) Lithium nitride is prepared by t ...
Amines - MCAT Cooperative
... Map of Carbon-Hydrogen framework (Can tell the protons and their environment) Sample in magnetic field, nuclei align with field. Bombard with electromagnetic energy, and at the resonance frequency, the nuclei turn to face against the magnetic field. Peak above 10=acid=free proton. Distinguish an ...
... Map of Carbon-Hydrogen framework (Can tell the protons and their environment) Sample in magnetic field, nuclei align with field. Bombard with electromagnetic energy, and at the resonance frequency, the nuclei turn to face against the magnetic field. Peak above 10=acid=free proton. Distinguish an ...
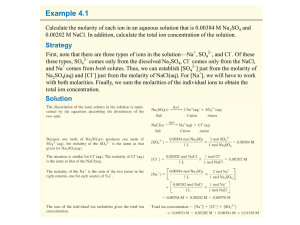
Chapter 9
... • Alcohols are converted to tosylates by treatment with ptoluenesulfonyl chloride (TsCl) in the presence of pyridine. • This process converts a poor leaving group (¯OH) into a good one (¯OTs). • Tosylate is a good leaving group because its conjugate acid, p-toluenesulfonic acid (CH3C6H4SO3H, TsOH) i ...
... • Alcohols are converted to tosylates by treatment with ptoluenesulfonyl chloride (TsCl) in the presence of pyridine. • This process converts a poor leaving group (¯OH) into a good one (¯OTs). • Tosylate is a good leaving group because its conjugate acid, p-toluenesulfonic acid (CH3C6H4SO3H, TsOH) i ...
Ethers and Epoxides - Delaware State University
... For a pure alkylthiol use thiourea (NH2(C=S)NH2) as the nucleophile This gives an intermediate alkylisothiourea salt, which is hydrolyzed cleanly to the alkyl thiourea ...
... For a pure alkylthiol use thiourea (NH2(C=S)NH2) as the nucleophile This gives an intermediate alkylisothiourea salt, which is hydrolyzed cleanly to the alkyl thiourea ...
solutions - chem.msu.su
... Then the molar fraction of the completely uncharged form of A is (0.5 point for each calculation of ...
... Then the molar fraction of the completely uncharged form of A is (0.5 point for each calculation of ...
Document
... (a) Because copper lies below hydrogen in the activity series of the metals, Cu(s) cannot reduce H+(aq) to H2(g) and be oxidized to Cu2+(aq). Looking at it the other way, H+ is not a strong enough oxidizing agent to oxidize Cu(s) to Cu 2+(aq). Chloride ion in HCl(aq) can only be a reducing agent. As ...
... (a) Because copper lies below hydrogen in the activity series of the metals, Cu(s) cannot reduce H+(aq) to H2(g) and be oxidized to Cu2+(aq). Looking at it the other way, H+ is not a strong enough oxidizing agent to oxidize Cu(s) to Cu 2+(aq). Chloride ion in HCl(aq) can only be a reducing agent. As ...
final review cp2 1213 by chapter
... ATB 6: What volume of 6 M HCl must be diluted to prepare 500 mL of 1.5 M HCl? ...
... ATB 6: What volume of 6 M HCl must be diluted to prepare 500 mL of 1.5 M HCl? ...
Topic 16 notes - A
... These two mirror images cannot be interconverted without breaking covalent bonds. Molecules which contain a carbon atom which is attached to four different groups are said to be chiral. Chiral molecules cannot be superimposed on their mirror image. The two non-superimposable mirror images are optica ...
... These two mirror images cannot be interconverted without breaking covalent bonds. Molecules which contain a carbon atom which is attached to four different groups are said to be chiral. Chiral molecules cannot be superimposed on their mirror image. The two non-superimposable mirror images are optica ...
Strychnine total synthesis

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field. At the time it formed the natural conclusion to an elaborate process of molecular structure elucidation that started with the isolation of strychnine from the beans of Strychnos ignatii by Pierre Joseph Pelletier and Joseph Bienaimé Caventou in 1818. Major contributors to the entire effort were Sir Robert Robinson with over 250 publications and Hermann Leuchs with another 125 papers in a time span of 40 years. Robinson was awarded the Nobel Prize in Chemistry in 1947 for his work on alkaloids, strychnine included. The process of chemical identification was completed with publications in 1946 by Robinson and later confirmed by Woodward in 1947. X-ray structures establishing the absolute configuration became available between 1947 and 1951 with publications from J. M. Bijvoet and J.H. Robertson .Woodward published a very brief account on the strychnine synthesis in 1954 (just 3 pages) and a lengthy one (42 pages) in 1963.Many more methods exist and reported by the research groups of Magnus, Overman, Kuehne, Rawal, Bosch, Vollhardt, Mori, Shibasaki, Li, Fukuyama Vanderwal and MacMillan. Synthetic (+)-strychnine is also known. Racemic synthesises were published by Padwa in 2007 and in 2010 by Andrade and by Reissig.In his 1963 publication Woodward quoted Sir Robert Robinson who said for its molecular size it is the most complex substance known.