Branched-Chain Alkanes
... carbon-carbon bond is shared almost equally by the nuclei of the atoms forming the bond. – Thus, hydrocarbons are nonpolar molecules. • The attractions between nonpolar molecules are weak van der Waals forces. • So, hydrocarbons with low molar masses tend to be gases or liquids that boil at a low te ...
... carbon-carbon bond is shared almost equally by the nuclei of the atoms forming the bond. – Thus, hydrocarbons are nonpolar molecules. • The attractions between nonpolar molecules are weak van der Waals forces. • So, hydrocarbons with low molar masses tend to be gases or liquids that boil at a low te ...
Coordination Chemistry Prof. Debashis Ray Department of
... just moving from one system to the other. And here also, we see that the connectivity is again through the four pyrole ring. And in some cases they are reduced, and this particular system when we can have a different metal center is utilized to bind. And this is the corresponding cobalt system. So, ...
... just moving from one system to the other. And here also, we see that the connectivity is again through the four pyrole ring. And in some cases they are reduced, and this particular system when we can have a different metal center is utilized to bind. And this is the corresponding cobalt system. So, ...
Fritz-Haber-Institut der Max-Planck
... reactivity and the deactivation behavior of unpromoted catalysts was performed. Excellent agreement was reached using basic adsorption data for EB, St and water from earlier measurements and adjusting the remaining parameters to measured reactivities. ...
... reactivity and the deactivation behavior of unpromoted catalysts was performed. Excellent agreement was reached using basic adsorption data for EB, St and water from earlier measurements and adjusting the remaining parameters to measured reactivities. ...
Chemistry 213 - Winona State University
... A series of trials will be performed on each complex in a calorimeter. A solution of one of the hexaaqua complex ion will be reacted with an equimolar amount of en. The heat of reaction will be determined from the increase in temperature of the solution. The process will be repeated until the additi ...
... A series of trials will be performed on each complex in a calorimeter. A solution of one of the hexaaqua complex ion will be reacted with an equimolar amount of en. The heat of reaction will be determined from the increase in temperature of the solution. The process will be repeated until the additi ...
Assignment 1
... burette has many graduation marks and, therefore, allows the volume of solution dispensed from it to be measured accurately and to the appropriate precision. P3. A measured volume of the weak base is placed in a conical flask. A strong acid of known concentration is added from a burette, with the pH ...
... burette has many graduation marks and, therefore, allows the volume of solution dispensed from it to be measured accurately and to the appropriate precision. P3. A measured volume of the weak base is placed in a conical flask. A strong acid of known concentration is added from a burette, with the pH ...
ation in Cytochrome P-450-Catalyzed Reactions
... isozymes and their respective mutants. These observations appear to be consistent only with the existence of two types of electrophilic oxidants in the P-450 reactions as previously described.8 One of the oxidants gives mainly methyl hydroxylation products, while the other gives mainly phenols from ...
... isozymes and their respective mutants. These observations appear to be consistent only with the existence of two types of electrophilic oxidants in the P-450 reactions as previously described.8 One of the oxidants gives mainly methyl hydroxylation products, while the other gives mainly phenols from ...
Palladium and Ruthenium Catalyzed Reactions By Bryan Jaksic
... catalyzed by a Pd(0)L2 species which are generated in situ from a palladium precatalyst and are often co-catalyzed by CuI although use of the latter is undesirable as it induces homocoupling in certain instances. The rate and quantity of active species generated is not known for the commonly used pr ...
... catalyzed by a Pd(0)L2 species which are generated in situ from a palladium precatalyst and are often co-catalyzed by CuI although use of the latter is undesirable as it induces homocoupling in certain instances. The rate and quantity of active species generated is not known for the commonly used pr ...
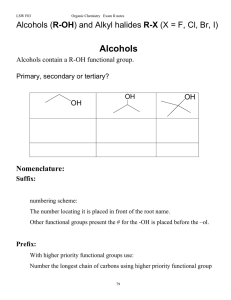
Alcohols (R-OH), and alkyl halides, RX
... With higher priority functional groups use: Number the longest chain of carbons using higher priority functional group ...
... With higher priority functional groups use: Number the longest chain of carbons using higher priority functional group ...
28 Coulomb`s Law: the equation Energyof electrostatic interaction
... is that the test reaction is being run in 85% ethanol, while the reference reaction, ionization of benzoic acids, was measured in pure water. Coulombs law tells us that electrostatic interactions are larger in less polar solvents. A significant part is that the addition to the carbonyl is really can ...
... is that the test reaction is being run in 85% ethanol, while the reference reaction, ionization of benzoic acids, was measured in pure water. Coulombs law tells us that electrostatic interactions are larger in less polar solvents. A significant part is that the addition to the carbonyl is really can ...
nucleophilic addition on ketones and ketimines - ISI
... The asymmetric allylation of ketones was also recently studied by the groups of Shibasaki22 and Yamamoto23 using soft transition metal–chiral diphosphine complexes as catalysts (Scheme 9). Shibasaki et al. used allyl boronates as nucleophiles for the allyl transfer on aliphatic and aromatic ketones ...
... The asymmetric allylation of ketones was also recently studied by the groups of Shibasaki22 and Yamamoto23 using soft transition metal–chiral diphosphine complexes as catalysts (Scheme 9). Shibasaki et al. used allyl boronates as nucleophiles for the allyl transfer on aliphatic and aromatic ketones ...
Chapter 24 Chemistry of Coordination Compounds
... Nomenclature of Coordination Compounds • As is the case with ionic compounds, the name of the cation appears first; the anion is named last. • Ligands are listed alphabetically before the metal. Prefixes denoting the number of a particular ligand are ignored when alphabetizing. ...
... Nomenclature of Coordination Compounds • As is the case with ionic compounds, the name of the cation appears first; the anion is named last. • Ligands are listed alphabetically before the metal. Prefixes denoting the number of a particular ligand are ignored when alphabetizing. ...
CHM 235 Course Outline and Homework in McMurry (6th ed.)
... CHM 235 Course Outline and Homework in McMurry (6th ed.) Fall 2006 Chapter 1: 1.19-1.26, 1.28-1.31, 1.34, 1.36-1.39, 1.46, 1.47 Atomic orbitals (s,p), electronic configuration (through calcium) Covalent vs ionic bonds, Lewis structures, valence electrons Valence bond theory, hybridization (sp3 ...
... CHM 235 Course Outline and Homework in McMurry (6th ed.) Fall 2006 Chapter 1: 1.19-1.26, 1.28-1.31, 1.34, 1.36-1.39, 1.46, 1.47 Atomic orbitals (s,p), electronic configuration (through calcium) Covalent vs ionic bonds, Lewis structures, valence electrons Valence bond theory, hybridization (sp3 ...
Redox reactions_organic - Welcome to SALEM
... Alkenes react rapidly and quantitatively with ozone form an unstable compound, known as ozonide ...
... Alkenes react rapidly and quantitatively with ozone form an unstable compound, known as ozonide ...
RESEARCH ARTICLE Coordination Chemistry of Cadmium
... Flavus. However in comparison their metal chelates have been shown to possess more antibacterial and antifungal activities than the uncomplexed Schiff bases. Key Words: Condensation, Screening, Uncomplexed, Schiff bases, Metal salt. INTRODUCTION Reaction between primary amines and aldehydes, forming ...
... Flavus. However in comparison their metal chelates have been shown to possess more antibacterial and antifungal activities than the uncomplexed Schiff bases. Key Words: Condensation, Screening, Uncomplexed, Schiff bases, Metal salt. INTRODUCTION Reaction between primary amines and aldehydes, forming ...
IOSR Journal of Applied Chemistry (IOSR-JAC)
... coupling reactions such as Heck,1 Suzuki,2 Kumada,3 Negishi,4 Stille,5 Hiyma,6 Sonogashira7 without a transition metal e.g. Pd, Ni etc. Major drawbacks of these catalysts are their toxicity, cost and low abundance. Hence these catalysts are unsuitable for the large-scale application, leaving aside t ...
... coupling reactions such as Heck,1 Suzuki,2 Kumada,3 Negishi,4 Stille,5 Hiyma,6 Sonogashira7 without a transition metal e.g. Pd, Ni etc. Major drawbacks of these catalysts are their toxicity, cost and low abundance. Hence these catalysts are unsuitable for the large-scale application, leaving aside t ...
Chapter 9
... Fluorine is too reactive to give mono-fluorinated products For Iodine, an oxidizing agent such as hydrogen peroxide or a copper salt such as CuCl2 must be added to the reaction • These substances oxidize I2 to the electrophilic species that reacts as if it were I+ • The aromatic ring reacts with the ...
... Fluorine is too reactive to give mono-fluorinated products For Iodine, an oxidizing agent such as hydrogen peroxide or a copper salt such as CuCl2 must be added to the reaction • These substances oxidize I2 to the electrophilic species that reacts as if it were I+ • The aromatic ring reacts with the ...
Hydroformylation

Hydroformylation, also known as oxo synthesis or oxo process, is an important homogeneously catalyzed industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention in 1938: Production capacity reached 6.6×106 tons in 1995. It is important because the resulting aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to plasticizers or detergents. Hydroformylation is also used in specialty chemicals, relevant to the organic synthesis of fragrances and natural products. The development of hydroformylation, which originated within the German coal-based industry, is considered one of the premier achievements of 20th-century industrial chemistry.The process typically entails treatment of an alkene with high pressures (between 10 to 100 atmospheres) of carbon monoxide and hydrogen at temperatures between 40 and 200 °C. Transition metal catalysts are required.