part three
... Oxidation numbers are hypothetical numbers assigned to an individual atom or ion present in a substance using a set of rules. Oxidation numbers can be positive, negative, or zero. It is VERY IMPORTANT to remember that oxidation numbers are always reported for one individual atom or ion and not for g ...
... Oxidation numbers are hypothetical numbers assigned to an individual atom or ion present in a substance using a set of rules. Oxidation numbers can be positive, negative, or zero. It is VERY IMPORTANT to remember that oxidation numbers are always reported for one individual atom or ion and not for g ...
12. 16 Physical Properties of Alkanes and Cycloalkanes.
... Be able to identify the steps on a potential diagram for a chemical reaction (pg. 121122). Be able to identify the polarity [nucleophiles (Nu:-)/eletrophiles (E+)], identify ...
... Be able to identify the steps on a potential diagram for a chemical reaction (pg. 121122). Be able to identify the polarity [nucleophiles (Nu:-)/eletrophiles (E+)], identify ...
The reaction pathways of hydrogen peroxide in
... breakdown of the intermediate have been calculated. The metal-catalyzed pathway of hydrogen peroxide is dealing with the effect of hydroxyl radicals created by the Fenton reaction and their potential to oxidize the disulfide bridge of small peptides. Based on the reduction potential, which is an ind ...
... breakdown of the intermediate have been calculated. The metal-catalyzed pathway of hydrogen peroxide is dealing with the effect of hydroxyl radicals created by the Fenton reaction and their potential to oxidize the disulfide bridge of small peptides. Based on the reduction potential, which is an ind ...
Introduction to Organometallic chemistry Prof. A. G. Samuelson
... So, the synthesis of the four membered rings, the cyclo butadiene rings, in the coordination sphere, the metal atom was done in a couple of different ways. And we will just look at a few ways, because it illustrates some different methods by which these molecules are synthesized. You can see here th ...
... So, the synthesis of the four membered rings, the cyclo butadiene rings, in the coordination sphere, the metal atom was done in a couple of different ways. And we will just look at a few ways, because it illustrates some different methods by which these molecules are synthesized. You can see here th ...
Chm_451_F-12_Exam_3_..
... 22b. (1 pt) Give an example of a ligand-to-metal charge transfer. You can show this with an arrow on the diagram above. 22c. (3 pts) We categorized transition metal complexes into three scenarios, the third of which was those compounds that could have only 18 electrons because the t2g orbitals shown ...
... 22b. (1 pt) Give an example of a ligand-to-metal charge transfer. You can show this with an arrow on the diagram above. 22c. (3 pts) We categorized transition metal complexes into three scenarios, the third of which was those compounds that could have only 18 electrons because the t2g orbitals shown ...
Microsoft Word - Open Access Repository of Indian Theses
... Figure 4 The synthesis of furanoid sugar amino acids, starting materials for the peptide analogs of 1, was described in Scheme 1-3. The starting material for the synthesis of Fmoc-protected 2 was furan carboxylic acid 13, which was prepared from L-glutamic acid 12 in 66% yield, following the reporte ...
... Figure 4 The synthesis of furanoid sugar amino acids, starting materials for the peptide analogs of 1, was described in Scheme 1-3. The starting material for the synthesis of Fmoc-protected 2 was furan carboxylic acid 13, which was prepared from L-glutamic acid 12 in 66% yield, following the reporte ...
Chapter 14 Review
... C. A catalyst speeds up the approach to equilibrium and shifts the position of equilibrium to the right. D. Decreasing the total pressure of the system shifts the equilibrium to the right. E. Increasing the temperature causes the equilibrium constant to increase. 38. The reaction 2NO(g) N2(g) + O2(g ...
... C. A catalyst speeds up the approach to equilibrium and shifts the position of equilibrium to the right. D. Decreasing the total pressure of the system shifts the equilibrium to the right. E. Increasing the temperature causes the equilibrium constant to increase. 38. The reaction 2NO(g) N2(g) + O2(g ...
Problem 28. TUNNELING IN CHEMISTRY
... Problem 1. ON THE BORDERS OF THE PERIODIC SYSTEM .................................................................................................. 4 Problem 2. SCHRÖDINGER CAT AND CHEMISTRY ............................................................................................................. ...
... Problem 1. ON THE BORDERS OF THE PERIODIC SYSTEM .................................................................................................. 4 Problem 2. SCHRÖDINGER CAT AND CHEMISTRY ............................................................................................................. ...
Chapter 4 Aqueous Reactions and Solution Stoichiometry
... dissolved in a solvent (water). 3. Solute = substance dissolved 4. Solvent = substance of greater quantity (usually water) ...
... dissolved in a solvent (water). 3. Solute = substance dissolved 4. Solvent = substance of greater quantity (usually water) ...
Table 2
... 16. Explain how [ PtCl2(NH3)2 ] and [ Pt(NH3)6 ] Cl4 will differ in their electrolytic conductance. Give the hybridization and oxidation states of Pt in these complexes. 17. Draw the structures of each of the following: (a) [ Cr(C2O4)3 ] 3(b) [Fe(CO)5] 18. Draw a figure to show the splitting of dege ...
... 16. Explain how [ PtCl2(NH3)2 ] and [ Pt(NH3)6 ] Cl4 will differ in their electrolytic conductance. Give the hybridization and oxidation states of Pt in these complexes. 17. Draw the structures of each of the following: (a) [ Cr(C2O4)3 ] 3(b) [Fe(CO)5] 18. Draw a figure to show the splitting of dege ...
Oxidative reactions ppt - Senior Chemistry
... You can’t pull the aldehyde out of this reaction, so the only product is the carboxylic acid. ...
... You can’t pull the aldehyde out of this reaction, so the only product is the carboxylic acid. ...
Chapter 16: Reaction Rates
... Collision orientation and the activated complex Why do most collisions fail to produce products? What other factors must be considered? Figure 16.4a and b show one possible answer to this question. These illustrations indicate that in order for a collision to lead to a reaction, the carbon atom in a ...
... Collision orientation and the activated complex Why do most collisions fail to produce products? What other factors must be considered? Figure 16.4a and b show one possible answer to this question. These illustrations indicate that in order for a collision to lead to a reaction, the carbon atom in a ...
Theoretical problems
... The acids which are stronger than pure sulfuric acid are called superacids. Superacids are very strong proton donors being capable of protonating even weak Lewis acids such as Xe, H2, Cl2, Br2, and CO2. Cations, which never exist in other media, have been observed in superacid solutions. George Olah ...
... The acids which are stronger than pure sulfuric acid are called superacids. Superacids are very strong proton donors being capable of protonating even weak Lewis acids such as Xe, H2, Cl2, Br2, and CO2. Cations, which never exist in other media, have been observed in superacid solutions. George Olah ...
Copper-Catalyzed Coupling Reactions Using Carbon
... Oxidative coupling reactions with C-H bonds are quite challenging due to the relative strong C-H bond, along with the associated selectivity issues with the myriad of C-H bonds that are available for the coupling reaction. ...
... Oxidative coupling reactions with C-H bonds are quite challenging due to the relative strong C-H bond, along with the associated selectivity issues with the myriad of C-H bonds that are available for the coupling reaction. ...
Microsoft Word - Open Access Repository of Indian Theses
... in zeolitic environment" has been divided in to three parts. Part I contains chapter I and which describes the introduction of the zeolites and photochemical reactions in zeolites. Part II is divided into two chapters. Chapter II deals with ionic photo-dissociation of arylallyl acetates through char ...
... in zeolitic environment" has been divided in to three parts. Part I contains chapter I and which describes the introduction of the zeolites and photochemical reactions in zeolites. Part II is divided into two chapters. Chapter II deals with ionic photo-dissociation of arylallyl acetates through char ...
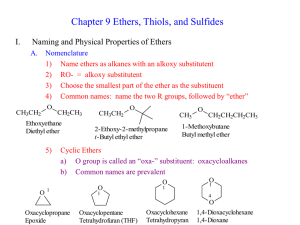
CHEM 210 Nomenclature Lecture 1
... substituent and proceed around the ring to give the second substituent the ...
... substituent and proceed around the ring to give the second substituent the ...
Chapter 22: Phenols. Alcohols contain an OH group bonded to an
... OH group of phenols can participate in hydrogen bonding with other phenol molecules and to water. 22.4: Acidity of Phenols. Phenols are more acidic than aliphatic alcohols pKa ~ 16 H3CH2C O H ...
... OH group of phenols can participate in hydrogen bonding with other phenol molecules and to water. 22.4: Acidity of Phenols. Phenols are more acidic than aliphatic alcohols pKa ~ 16 H3CH2C O H ...
Hydroformylation

Hydroformylation, also known as oxo synthesis or oxo process, is an important homogeneously catalyzed industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention in 1938: Production capacity reached 6.6×106 tons in 1995. It is important because the resulting aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to plasticizers or detergents. Hydroformylation is also used in specialty chemicals, relevant to the organic synthesis of fragrances and natural products. The development of hydroformylation, which originated within the German coal-based industry, is considered one of the premier achievements of 20th-century industrial chemistry.The process typically entails treatment of an alkene with high pressures (between 10 to 100 atmospheres) of carbon monoxide and hydrogen at temperatures between 40 and 200 °C. Transition metal catalysts are required.



![Imidazole-Based [2 + 1] Re(I)/99mTc(I](http://s1.studyres.com/store/data/016453198_1-7284afe0756f420c3d5e168652a9a305-300x300.png)