manuscript (2)
... barrier for oxygen migration [12-15]. Iron is an interesting dopant, especially because of its own redox behaviour (Fe3+/Fe2+). Zn as well has been used as a CeO2-doping metal and a positive effect of its addition on the catalytic performance have been reported [16]. However, Ce-Zn solid solution fo ...
... barrier for oxygen migration [12-15]. Iron is an interesting dopant, especially because of its own redox behaviour (Fe3+/Fe2+). Zn as well has been used as a CeO2-doping metal and a positive effect of its addition on the catalytic performance have been reported [16]. However, Ce-Zn solid solution fo ...
50 chemistry questions for class xii
... For example- heart has more β adrenergic receptors than α adrenergic receptors .This means that a drug designed to interact with β adrenergic receptors will act on heart rather than a tissue which are rich in α adrenergic receptors . Q49 Define the following terms with one example(Any two) (a) Antip ...
... For example- heart has more β adrenergic receptors than α adrenergic receptors .This means that a drug designed to interact with β adrenergic receptors will act on heart rather than a tissue which are rich in α adrenergic receptors . Q49 Define the following terms with one example(Any two) (a) Antip ...
STOICHIOMETRY via ChemLog - Small
... When carrying out a chemical reaction, we may use the exact amount of each reactant needed. Or, we may use an excess of some reactants and a limited amount of others. We may do this if one reactant is very expensive and others are inexpensive so that we can use all of the expensive compound. It can ...
... When carrying out a chemical reaction, we may use the exact amount of each reactant needed. Or, we may use an excess of some reactants and a limited amount of others. We may do this if one reactant is very expensive and others are inexpensive so that we can use all of the expensive compound. It can ...
Influence of Substituents on the Energy and Nature of the Lowest
... 3.1. Nature of the Frontier Molecular Orbitals. The influence of a particular substituent on an aromatic system is usually discussed in terms of inductive and mesomeric effects. The mesomeric effect is related to the sharing of π-electrons between the aromatic core and the substituent. If the substi ...
... 3.1. Nature of the Frontier Molecular Orbitals. The influence of a particular substituent on an aromatic system is usually discussed in terms of inductive and mesomeric effects. The mesomeric effect is related to the sharing of π-electrons between the aromatic core and the substituent. If the substi ...
irm_ch25
... 25.21 One ATP molecule is expended in the conversion of glycerol to a glycolysis intermediate; the conversion of glycerol to glycerol 3-phosphate uses one ATP molecule in the phosphorylation. 25.22 converted to pyruvate (glycolysis) or glucose (gluconeogenesis) 25.23 The site of fatty acid activatio ...
... 25.21 One ATP molecule is expended in the conversion of glycerol to a glycolysis intermediate; the conversion of glycerol to glycerol 3-phosphate uses one ATP molecule in the phosphorylation. 25.22 converted to pyruvate (glycolysis) or glucose (gluconeogenesis) 25.23 The site of fatty acid activatio ...
Fe(H 2 O) 6 2+ + Fe(H 2 O) 6 3+ *Fe(H 2 O) 6 3+ + Fe(H 2 O) 6 2+
... • Redox reactions are very important in inorganic and bioinorganic chemistry. The shuttling of electrons between transition metal cations is at the centre of a wide variety of vital biological processes • Redox reactions involving transition metal complexes generally occur very rapidly: thermodyna ...
... • Redox reactions are very important in inorganic and bioinorganic chemistry. The shuttling of electrons between transition metal cations is at the centre of a wide variety of vital biological processes • Redox reactions involving transition metal complexes generally occur very rapidly: thermodyna ...
Lecture - Ch 21
... – Symmetrical anhydrides of unsubstituted monocarboxylic acids and cyclic anhydrides of dicarboxylic acids are named by replacing acid with anhydride – Unsymmetrical anhydrides are named by listing the two acids alphabetically and then adding ...
... – Symmetrical anhydrides of unsubstituted monocarboxylic acids and cyclic anhydrides of dicarboxylic acids are named by replacing acid with anhydride – Unsymmetrical anhydrides are named by listing the two acids alphabetically and then adding ...
CH 223 Chapter 19 Lecture Notes
... Crystal Field Theory Electrostatic attractions between the positive metal ion and the negative ligands bond them together, lowering the energy of the whole system The lone pair e-’s on ligands repulsed by e-’s in metal d orbitals; this interaction is called the crystal field The repulsion influences ...
... Crystal Field Theory Electrostatic attractions between the positive metal ion and the negative ligands bond them together, lowering the energy of the whole system The lone pair e-’s on ligands repulsed by e-’s in metal d orbitals; this interaction is called the crystal field The repulsion influences ...
Supporting Information for Angew. Chem. Int. Ed. Z52444 © Wiley
... stirred at room temperature for 15 min. Powdered anhydrous Cs2CO3 (0.4 mmol, 0.4 equiv.) and a chloroform solution (2.0 mL) of the alcohol (1.0 mmol, 1.0 equiv) was introduced and the reaction mixture was maintained at room temperature. The reaction was monitored by standard analytical techniques (T ...
... stirred at room temperature for 15 min. Powdered anhydrous Cs2CO3 (0.4 mmol, 0.4 equiv.) and a chloroform solution (2.0 mL) of the alcohol (1.0 mmol, 1.0 equiv) was introduced and the reaction mixture was maintained at room temperature. The reaction was monitored by standard analytical techniques (T ...
Thermochemistry (4 lectures)
... Ligand substitution reactions • Dissociative and associative mechanisms possible • Rates vary widely for transition metal complexes M3+ slower than M2+ dn with large LFSE are slow (d3, d8 and low spin d5-7) Today’s lecture • e- transfer reactions ...
... Ligand substitution reactions • Dissociative and associative mechanisms possible • Rates vary widely for transition metal complexes M3+ slower than M2+ dn with large LFSE are slow (d3, d8 and low spin d5-7) Today’s lecture • e- transfer reactions ...
Chapter 25 Organic and Biological Chemistry
... • The boiling point increases with the length Biological Chemistry of the chain. © 2009, Prentice-Hall, Inc. ...
... • The boiling point increases with the length Biological Chemistry of the chain. © 2009, Prentice-Hall, Inc. ...
PDF (Chapter1)
... Examination of the literature of phosphine-supported transition metal chemistry shows an abundance of cationic complexes that are used for spectroscopic studies, stoichiometric transformations, and catalytic conversions.10,11 Many of these cationic complexes result from multidentate, neutral phosphi ...
... Examination of the literature of phosphine-supported transition metal chemistry shows an abundance of cationic complexes that are used for spectroscopic studies, stoichiometric transformations, and catalytic conversions.10,11 Many of these cationic complexes result from multidentate, neutral phosphi ...
Chapter 8
... formed from reactant A, assuming it is the limiting reactant b) Find the number of moles of product that can be formed from reactant B, assuming it is the limiting reactant 3) Whichever number of moles of product is smallest corresponds to the limiting reactant. The other reactant is in excess. The ...
... formed from reactant A, assuming it is the limiting reactant b) Find the number of moles of product that can be formed from reactant B, assuming it is the limiting reactant 3) Whichever number of moles of product is smallest corresponds to the limiting reactant. The other reactant is in excess. The ...
Substitution Reactions of Specifically Ortho
... with a serum cap was placed 2 mmol of imine in 15 ml of THF and the mixture was cooled to -78 "C with a Coyacetone bath. To the stirred solution was added a solution of 2.1 mmol of n-BuLi-hexane (AlfaVentron) over 1min via syringe through the serum cap. A yellow color appeared upon introduction of t ...
... with a serum cap was placed 2 mmol of imine in 15 ml of THF and the mixture was cooled to -78 "C with a Coyacetone bath. To the stirred solution was added a solution of 2.1 mmol of n-BuLi-hexane (AlfaVentron) over 1min via syringe through the serum cap. A yellow color appeared upon introduction of t ...
Synthesis of Molybdenum Alkylidene Complexes That Contain the 2,6-Dimesitylphenylimido Ligand
... have been prepared and explored as olefin metathesis initiators; examples are 1, 2, and 3.1,2 These MonoAlkoxidePyrrolide (MAP) species have been found to be efficient for many Z-selective reactions. It has been proposed that Z selectivity arises as a consequence of a “large” phenoxide, especially in c ...
... have been prepared and explored as olefin metathesis initiators; examples are 1, 2, and 3.1,2 These MonoAlkoxidePyrrolide (MAP) species have been found to be efficient for many Z-selective reactions. It has been proposed that Z selectivity arises as a consequence of a “large” phenoxide, especially in c ...
Recent developments in the synthesis of functional poly(olefin)s
... Functionalization of poly(ole®n)s especially poly(propylene), poly(ethylene) and poly(ethylene-copropylene) has been extensively investigated using melt phase free radical grafting. The synthetic technique involves activation of the polymer by shear in presence of free radical initiators or high ene ...
... Functionalization of poly(ole®n)s especially poly(propylene), poly(ethylene) and poly(ethylene-copropylene) has been extensively investigated using melt phase free radical grafting. The synthetic technique involves activation of the polymer by shear in presence of free radical initiators or high ene ...
lab 15: hydrocarbons
... 5. On the report sheet make a graph of the total mass lost by each substance every 15 minutes. Draw a straight line through the graph points to show the linear relationship for the evaporation of each liquid. Compare to determine relative volatility.5 ...
... 5. On the report sheet make a graph of the total mass lost by each substance every 15 minutes. Draw a straight line through the graph points to show the linear relationship for the evaporation of each liquid. Compare to determine relative volatility.5 ...
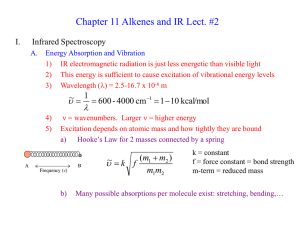
Chapter 1 Structure and Bonding
... Hyperconjugation makes substituted alkenes more stable by stabilizing p-orbitals cis alkenes are less stable than trans alkenes because of steric crowding cis cycloalkenes are more stable than trans for the small rings ...
... Hyperconjugation makes substituted alkenes more stable by stabilizing p-orbitals cis alkenes are less stable than trans alkenes because of steric crowding cis cycloalkenes are more stable than trans for the small rings ...
carbonyl compounds
... They react with carbonates and the fizzing produced can be used to distinguish carboxylic acids from other organic compounds 2CH3COOH + CaCO3 (CH3COO)2Ca + H2O + CO2 Ethanoic acid calcium ethanoate ...
... They react with carbonates and the fizzing produced can be used to distinguish carboxylic acids from other organic compounds 2CH3COOH + CaCO3 (CH3COO)2Ca + H2O + CO2 Ethanoic acid calcium ethanoate ...
Corynebactin and Enterobactin: Related Siderophores of Opposite
... Gram-negative4a,b and Gram-positive4c bacteria, and has an extraordinarily high stability (Kf ) 1049)5 with metal coordination at neutral pH accomplished through the six catecholate oxygens.6 The chirality of the iron center in enterobactin is ∆,6 and this chirality, while not essential for receptor ...
... Gram-negative4a,b and Gram-positive4c bacteria, and has an extraordinarily high stability (Kf ) 1049)5 with metal coordination at neutral pH accomplished through the six catecholate oxygens.6 The chirality of the iron center in enterobactin is ∆,6 and this chirality, while not essential for receptor ...
Introduction_Coordination_Chemistry IBChemLab
... of the brackets is present for charge balance. Therefore the formulas [Co(H2O)6]Cl3 and [Ni(H2O)6]Cl3 represent neutral compounds in which the chloride ions are present for charge balance. The formulas K3[Co(NO2)6] and K4[Fe(CN)6] represent molecules containing negatively charged complex ions with p ...
... of the brackets is present for charge balance. Therefore the formulas [Co(H2O)6]Cl3 and [Ni(H2O)6]Cl3 represent neutral compounds in which the chloride ions are present for charge balance. The formulas K3[Co(NO2)6] and K4[Fe(CN)6] represent molecules containing negatively charged complex ions with p ...
Alcohols phenols
... LAH is much stronger reducing agent than NaBH4. However, NaBH4 is more selective and does not reduce less active carbonyl group in acids, esters and amides. Hence, the ketonic and aldehydic group can be selectively reduced in the presence of an acid or an ester group using NaBH4. ...
... LAH is much stronger reducing agent than NaBH4. However, NaBH4 is more selective and does not reduce less active carbonyl group in acids, esters and amides. Hence, the ketonic and aldehydic group can be selectively reduced in the presence of an acid or an ester group using NaBH4. ...
Hydroformylation

Hydroformylation, also known as oxo synthesis or oxo process, is an important homogeneously catalyzed industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention in 1938: Production capacity reached 6.6×106 tons in 1995. It is important because the resulting aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to plasticizers or detergents. Hydroformylation is also used in specialty chemicals, relevant to the organic synthesis of fragrances and natural products. The development of hydroformylation, which originated within the German coal-based industry, is considered one of the premier achievements of 20th-century industrial chemistry.The process typically entails treatment of an alkene with high pressures (between 10 to 100 atmospheres) of carbon monoxide and hydrogen at temperatures between 40 and 200 °C. Transition metal catalysts are required.