Potassium Ferric Oxalate
... It should be noted our product crystallizes as a Hydrate. Hydrates are salts that crystallize from a water solution and contain weakly bound water molecules. For example, Ferric Chloride, FeCl3, crystallizes with six water molecules weakly bound to the Fe3+ ion in an octahedral arrangement: ...
... It should be noted our product crystallizes as a Hydrate. Hydrates are salts that crystallize from a water solution and contain weakly bound water molecules. For example, Ferric Chloride, FeCl3, crystallizes with six water molecules weakly bound to the Fe3+ ion in an octahedral arrangement: ...
constitutional isomers
... Note that ethers are not on this table because there is no suffix for ethers. Alkenes and alkynes are considered ‘less important’ than all functional groups in this table. ...
... Note that ethers are not on this table because there is no suffix for ethers. Alkenes and alkynes are considered ‘less important’ than all functional groups in this table. ...
Chem 342 Jasperse Syllabus 1 Organic Chemistry II READING
... What’s Covered in Organic I versus Organic II differs between NDSU and MSUM The following are reading sections and problems associated with one chapter that was covered at MSUM in Organic I but is covered at NDSU in Organic II. Thus, if you are an NDSU student taking Organic II at MSUM, you will end ...
... What’s Covered in Organic I versus Organic II differs between NDSU and MSUM The following are reading sections and problems associated with one chapter that was covered at MSUM in Organic I but is covered at NDSU in Organic II. Thus, if you are an NDSU student taking Organic II at MSUM, you will end ...
101. Alcohols as alkylating agents in heteroarene C H functionalization
... Redox processes and radical intermediates are found in many biochemical processes, including deoxyribonucleotide synthesis and oxidative DNA damage1. One of the core principles underlying DNA biosynthesis is the radical-mediated elimination of H2O to deoxygenate ribonucleotides, an example of ‘spin- ...
... Redox processes and radical intermediates are found in many biochemical processes, including deoxyribonucleotide synthesis and oxidative DNA damage1. One of the core principles underlying DNA biosynthesis is the radical-mediated elimination of H2O to deoxygenate ribonucleotides, an example of ‘spin- ...
Chapter 24 Chemistry of Coordination Compounds
... 1893 that metal ions exhibit what he called primary and secondary valences. Primary valences were the oxidation number for the metal (+3 on the cobalt at the right). Secondary valences were the coordination number, the number of atoms directly bonded to the metal (6 in the complex at the right). ...
... 1893 that metal ions exhibit what he called primary and secondary valences. Primary valences were the oxidation number for the metal (+3 on the cobalt at the right). Secondary valences were the coordination number, the number of atoms directly bonded to the metal (6 in the complex at the right). ...
Complexometric Reactions (1)
... If the equilibrium is in the opposite direction, the constant is the reciprocal of the formation constant and is called the dissociation constant: ...
... If the equilibrium is in the opposite direction, the constant is the reciprocal of the formation constant and is called the dissociation constant: ...
Catalysis: from single crystals to the “real world”
... of promoters/ inhibitors on catalytic activity, and, in certain cases, the identification of reaction intermediates by post-reaction surface analysis. These kinds of studies have established the validity of using single-crystal surfaces as models for the more complex technical catalysts for a variet ...
... of promoters/ inhibitors on catalytic activity, and, in certain cases, the identification of reaction intermediates by post-reaction surface analysis. These kinds of studies have established the validity of using single-crystal surfaces as models for the more complex technical catalysts for a variet ...
07.3 - Reactions in aqueous solutions
... electrons), we say that it is reduced. The gain of electrons by a substance is called reduction. When one reactant loses electrons (when it is oxidized), another reactant must gain them. In other words, oxidation of one substance must be accompanied by reduction of some other substance. ...
... electrons), we say that it is reduced. The gain of electrons by a substance is called reduction. When one reactant loses electrons (when it is oxidized), another reactant must gain them. In other words, oxidation of one substance must be accompanied by reduction of some other substance. ...
platinum metals review - Johnson Matthey Technology Review
... of too mumin and vented through a bubbler acting as a gas seal. The reaction flask contents were stirred by a magnetic stirrer at 600 rpm. Typically, 0.1 g 1 0 per cent palladium on carbon catalyst was used. Under these conditions the reactions studied are under gas mass transfer control where the r ...
... of too mumin and vented through a bubbler acting as a gas seal. The reaction flask contents were stirred by a magnetic stirrer at 600 rpm. Typically, 0.1 g 1 0 per cent palladium on carbon catalyst was used. Under these conditions the reactions studied are under gas mass transfer control where the r ...
M(pic)2 Experiment
... in the periodic table (named the coinage metals along with gold), but their chemical properties are quite different. Although Cu(II) is the stable oxidation state in aqueous solution, Ag(II) is powerfully oxidizing in water. The conjugate base of picH, pyridine–2–carboxylate or picolinate ion, acts ...
... in the periodic table (named the coinage metals along with gold), but their chemical properties are quite different. Although Cu(II) is the stable oxidation state in aqueous solution, Ag(II) is powerfully oxidizing in water. The conjugate base of picH, pyridine–2–carboxylate or picolinate ion, acts ...
20 More About Oxidation–Reduction Reactions
... that these three ways to describe H 2 correspond to the three mechanisms by which H 2 is added to an organic compound. components of H:H ...
... that these three ways to describe H 2 correspond to the three mechanisms by which H 2 is added to an organic compound. components of H:H ...
A Voyage through Equations
... 2Na +Cl2 2NaCl 2. When solid copper reacts with aqueous silver nitrate, the products are aqueous copper(II) nitrate and silver metal. Cu + 2AgNO3 Cu(NO3)2 + 2Ag 3. Solid iron (III) oxide and carbon monoxide react to produce iron metal and carbon dioxide gas. Fe2O3 + 3CO 2Fe + 3CO2 4. Sulfuric ...
... 2Na +Cl2 2NaCl 2. When solid copper reacts with aqueous silver nitrate, the products are aqueous copper(II) nitrate and silver metal. Cu + 2AgNO3 Cu(NO3)2 + 2Ag 3. Solid iron (III) oxide and carbon monoxide react to produce iron metal and carbon dioxide gas. Fe2O3 + 3CO 2Fe + 3CO2 4. Sulfuric ...
SAMPLE PROBLEM
... Another feasible stereoselective retrosynthesis involves net addition of two nitrogen atoms across the double bond which should generate structure 8 from 9.1 The diastereoselectivity is controlled at this point but there will be a racemic mixture (two enantiomers in the reaction vessel) once the syn ...
... Another feasible stereoselective retrosynthesis involves net addition of two nitrogen atoms across the double bond which should generate structure 8 from 9.1 The diastereoselectivity is controlled at this point but there will be a racemic mixture (two enantiomers in the reaction vessel) once the syn ...
Lecture 3-edited
... 1.3.2 Chromic Acid Oxidation (Jones oxidation) The combination of CrO3 and sulfuric acid is often referred as Jones reagent, and the oxidation of alcohols with this reagent in acetone is called Jones oxidation. The reagent is selective as it is useful for the oxidation of alcohols, which contain car ...
... 1.3.2 Chromic Acid Oxidation (Jones oxidation) The combination of CrO3 and sulfuric acid is often referred as Jones reagent, and the oxidation of alcohols with this reagent in acetone is called Jones oxidation. The reagent is selective as it is useful for the oxidation of alcohols, which contain car ...
Chapter 5
... The metal promoted carbonylative cycloaddition reaction involving one alkene, one alkyne and carbon monoxide is one of the most convergent means for synthesizing cyclopentenone skeletons.1 The cyclopentenone formation reaction can be regarded as a formal [2+2+1] cycloaddition in which the alkyne and ...
... The metal promoted carbonylative cycloaddition reaction involving one alkene, one alkyne and carbon monoxide is one of the most convergent means for synthesizing cyclopentenone skeletons.1 The cyclopentenone formation reaction can be regarded as a formal [2+2+1] cycloaddition in which the alkyne and ...
14_11_15.html
... combination of TiCl4 and (CH3CH2)2AlCl, or TiCl3 and (CH3CH2)3Al. Many Ziegler-Natta catalyst combinations include a metallocene. ...
... combination of TiCl4 and (CH3CH2)2AlCl, or TiCl3 and (CH3CH2)3Al. Many Ziegler-Natta catalyst combinations include a metallocene. ...
Alcohols and Phenols
... Phenols (pKa ~10) are much more acidic than alcohols (pKa ~ 16) because of resonance stabilization of the phenoxide ion Phenols react with NaOH solutions (but alcohols do not), forming salts that are soluble in dilute aqueous solution A phenolic component can be separated from an organic solution by ...
... Phenols (pKa ~10) are much more acidic than alcohols (pKa ~ 16) because of resonance stabilization of the phenoxide ion Phenols react with NaOH solutions (but alcohols do not), forming salts that are soluble in dilute aqueous solution A phenolic component can be separated from an organic solution by ...
CHEMISTRY 263
... From TWG Solomons and CB Fryhle "Organic Chemistry" 8th Edition (2004): Functional Group List pp 70-71 and Periodic Table (1 page back from Back Cover) Chapter 6 – Substitution Reactions (re-read alcohols from alkyl halides) Chapter 8 – Alkenes (re-read alcohols from alkenes) Chapter 11 – Al ...
... From TWG Solomons and CB Fryhle "Organic Chemistry" 8th Edition (2004): Functional Group List pp 70-71 and Periodic Table (1 page back from Back Cover) Chapter 6 – Substitution Reactions (re-read alcohols from alkyl halides) Chapter 8 – Alkenes (re-read alcohols from alkenes) Chapter 11 – Al ...
Organic chemistry involves the study of the structures, properties
... The current major advances in Organic Chemistry technology concerns the synthesis of (1) novel polymers for highly specific uses, (2) drugs initially "designed" by molecular modeling & synthesized by combinatorial methods. (3) Rare or complex biologically important compounds (such as proteins and g ...
... The current major advances in Organic Chemistry technology concerns the synthesis of (1) novel polymers for highly specific uses, (2) drugs initially "designed" by molecular modeling & synthesized by combinatorial methods. (3) Rare or complex biologically important compounds (such as proteins and g ...
Lecture notes for chapter 6
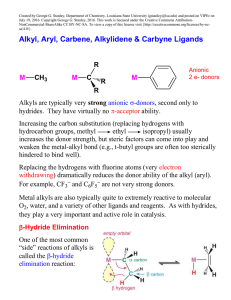
... aryl complexes are usually relatively stable compared to alkyls with hydrogens. But “stable” is a relative term since transition metal aryl complexes are usually quite air-sensitive. Aryls do have the potential for both -donation and -backbonding through the filled aryl -orbitals and empty * an ...
... aryl complexes are usually relatively stable compared to alkyls with hydrogens. But “stable” is a relative term since transition metal aryl complexes are usually quite air-sensitive. Aryls do have the potential for both -donation and -backbonding through the filled aryl -orbitals and empty * an ...
Reactions of carbohydrates
... • React the sugar with alcohol in acid. • Since the open chain sugar is in equilibrium with its - and -hemiacetal, both anomers of the acetal are formed. • Aglycone is the term used for the group bonded to the anomeric carbon. ...
... • React the sugar with alcohol in acid. • Since the open chain sugar is in equilibrium with its - and -hemiacetal, both anomers of the acetal are formed. • Aglycone is the term used for the group bonded to the anomeric carbon. ...
Hydroformylation

Hydroformylation, also known as oxo synthesis or oxo process, is an important homogeneously catalyzed industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention in 1938: Production capacity reached 6.6×106 tons in 1995. It is important because the resulting aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to plasticizers or detergents. Hydroformylation is also used in specialty chemicals, relevant to the organic synthesis of fragrances and natural products. The development of hydroformylation, which originated within the German coal-based industry, is considered one of the premier achievements of 20th-century industrial chemistry.The process typically entails treatment of an alkene with high pressures (between 10 to 100 atmospheres) of carbon monoxide and hydrogen at temperatures between 40 and 200 °C. Transition metal catalysts are required.