Chapter_23_Transition_Metal_Chemistry
... (neutral), and a Pt ion with an oxidation number of +4. The net charge on the cation must be +2, [Pt(en)2Cl2]2+. Two nitrate ions are needed to balance the +2 charge of the complex cation. Therefore, the formula of the compound is [Pt(en)2Cl2](NO3)2 . (c) The complex anion contains six nitro groups ...
... (neutral), and a Pt ion with an oxidation number of +4. The net charge on the cation must be +2, [Pt(en)2Cl2]2+. Two nitrate ions are needed to balance the +2 charge of the complex cation. Therefore, the formula of the compound is [Pt(en)2Cl2](NO3)2 . (c) The complex anion contains six nitro groups ...
Exam 2 Review A
... 2. Be able to write a definition of Markovnikov’s rule, and employ the Hammond-Leffler postulate in an explanation of the regioselective addition of HX to an unsymmetrical alkene. 3. Be able to explain how alkenes can be hydrated with water under acidic conditions, using an arrow-pushing mechanism t ...
... 2. Be able to write a definition of Markovnikov’s rule, and employ the Hammond-Leffler postulate in an explanation of the regioselective addition of HX to an unsymmetrical alkene. 3. Be able to explain how alkenes can be hydrated with water under acidic conditions, using an arrow-pushing mechanism t ...
elements of chemistry unit
... Although the two compounds above have the same molecular formula, their structural formulas are different in the way that the 4 carbons are assembled. As seen below, structure is just as essential as composition to understanding organic chemistry. C4H10 ISOMERS The two varieties of C4H10 shown are i ...
... Although the two compounds above have the same molecular formula, their structural formulas are different in the way that the 4 carbons are assembled. As seen below, structure is just as essential as composition to understanding organic chemistry. C4H10 ISOMERS The two varieties of C4H10 shown are i ...
Visible light photooxidation of nitrate: the dawn of
... LiNO3, diketone 3 and ketone 4 were obtained after 2 h of irradiation with blue light (l = 455 nm) with yields comparable to previous methods.27 When oxygen was replaced by ammonium persulfate as the electron acceptor in a degassed system, the yield and product ratio were not changed significantly ( ...
... LiNO3, diketone 3 and ketone 4 were obtained after 2 h of irradiation with blue light (l = 455 nm) with yields comparable to previous methods.27 When oxygen was replaced by ammonium persulfate as the electron acceptor in a degassed system, the yield and product ratio were not changed significantly ( ...
the optimization of proton exchange membrane hydrogen fuel cells
... A catalyst that has ideal selectivity minimizes the number of intermediates and unwanted waste products in the reactions at the anode and cathode [9]. The hydrogen oxidation has only one possible mechanism and therefore the selectivity does not affect the anode [9]. There are two possible mechanisms ...
... A catalyst that has ideal selectivity minimizes the number of intermediates and unwanted waste products in the reactions at the anode and cathode [9]. The hydrogen oxidation has only one possible mechanism and therefore the selectivity does not affect the anode [9]. There are two possible mechanisms ...
4. chemical kinetics
... Not only the time taken for the initial concentration is to reach half its value but the time taken for it to reach any fraction (1/4 or 3/4) of the initial concentration is independent of initial concentration. This is one of the main characteristics of a first order reaction. ...
... Not only the time taken for the initial concentration is to reach half its value but the time taken for it to reach any fraction (1/4 or 3/4) of the initial concentration is independent of initial concentration. This is one of the main characteristics of a first order reaction. ...
Ester Lab - Parkway C-2
... The R and R represent alkyl groups such as methyl, ethyl, or propyl. The esters are named after the compounds from which they are formed. The first part of the name comes from the alcohol, and the second part of the name comes from the carboxylic acid, with the “oic” changed to “oate”. Thus when eth ...
... The R and R represent alkyl groups such as methyl, ethyl, or propyl. The esters are named after the compounds from which they are formed. The first part of the name comes from the alcohol, and the second part of the name comes from the carboxylic acid, with the “oic” changed to “oate”. Thus when eth ...
UNIT 4
... This is reduced to silver when an aldehyde is added. Fehling’s Solution This is a blue solution containing Cu2+ ions. When aldehydes are added and heated, these ions are reduced to cooper (I) oxide Cu2O, producing a brick-red ppt. AQA A2 text book, p64 ...
... This is reduced to silver when an aldehyde is added. Fehling’s Solution This is a blue solution containing Cu2+ ions. When aldehydes are added and heated, these ions are reduced to cooper (I) oxide Cu2O, producing a brick-red ppt. AQA A2 text book, p64 ...
Carbohydrate-Metal Interactions Shaped by Supramolecular
... The side of the square is not long enough to accommodate the sodium ions into the square plane, thus they are located outside the plane. Of the two possible orientations (inside and outside the double torus), the inside-the-double-torus arrangement is realized and the homodromic hexaqua cycle can fo ...
... The side of the square is not long enough to accommodate the sodium ions into the square plane, thus they are located outside the plane. Of the two possible orientations (inside and outside the double torus), the inside-the-double-torus arrangement is realized and the homodromic hexaqua cycle can fo ...
Complexometric Reactions and Titrations
... an electron rich, and thus, electron donating species. A metal will thus accept electrons from a ligand where coordination bonds are formed. Electrons forming coordination bonds come solely from ligands. ...
... an electron rich, and thus, electron donating species. A metal will thus accept electrons from a ligand where coordination bonds are formed. Electrons forming coordination bonds come solely from ligands. ...
Standard enthalpies of of calcium, magnesium
... This is in accordance with the structure of aluminosalicylic acid. Considcring the two M - 0 bonds in each case as equivalent, the mean molar-bond dissociation enthalpy in the compound can be defined as half the molar enthalpy of the disruption reaction. In other words, the heats of chelation in the ...
... This is in accordance with the structure of aluminosalicylic acid. Considcring the two M - 0 bonds in each case as equivalent, the mean molar-bond dissociation enthalpy in the compound can be defined as half the molar enthalpy of the disruption reaction. In other words, the heats of chelation in the ...
Document
... NH4HS(s) = NH3(g) + H2S(g) a. Some solid NH4HS is placed in an evacuated vessel at 25°VC. After equilibrium is attained, the total pressure inside the vessel is found to be 0.659 atmospheres. Some solid NH4HS remains in the vessel at equilibrium. For this decomposition, write the expression for KP a ...
... NH4HS(s) = NH3(g) + H2S(g) a. Some solid NH4HS is placed in an evacuated vessel at 25°VC. After equilibrium is attained, the total pressure inside the vessel is found to be 0.659 atmospheres. Some solid NH4HS remains in the vessel at equilibrium. For this decomposition, write the expression for KP a ...
CHAPtER 9 Properties and reactions of organic compounds
... Some molecules can be described as chiral. They have what is known as an asymmetric carbon atom or chiral centre; this means that they cannot be superimposed on their mirror images. We are familiar with many chiral objects. For example, seashells, scissors, gloves, hands and feet cannot be matched d ...
... Some molecules can be described as chiral. They have what is known as an asymmetric carbon atom or chiral centre; this means that they cannot be superimposed on their mirror images. We are familiar with many chiral objects. For example, seashells, scissors, gloves, hands and feet cannot be matched d ...
IOSR Journal of Applied Chemistry (IOSR-JAC) e-ISSN: 2278-5736.
... Multiple Equilibria and Chemical distribution of some Bio metals with β-amide αWhere M1 (II) and M2 (II) are Co / Ni / Cu and Zn A = Primary ligand i.e. Asparagine and B = Secondary ligand i.e. Thymine . Each set of solution was then titrated against alkali (NaOH). The pH meter reading with progres ...
... Multiple Equilibria and Chemical distribution of some Bio metals with β-amide αWhere M1 (II) and M2 (II) are Co / Ni / Cu and Zn A = Primary ligand i.e. Asparagine and B = Secondary ligand i.e. Thymine . Each set of solution was then titrated against alkali (NaOH). The pH meter reading with progres ...
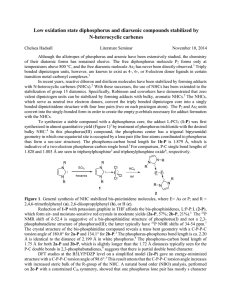
Low oxidation state diphosphorus and diarsenic compounds
... Although the allotropes of phosphorus and arsenic have been extensively studied, the chemistry of their diatomic forms has remained elusive. The free diphosphorus molecule P2 forms only at temperatures above 800 °C, and the free diarsenic molecule As2 has never been directly observed.1 Triply bonded ...
... Although the allotropes of phosphorus and arsenic have been extensively studied, the chemistry of their diatomic forms has remained elusive. The free diphosphorus molecule P2 forms only at temperatures above 800 °C, and the free diarsenic molecule As2 has never been directly observed.1 Triply bonded ...
Molecular Water Oxidation Catalysts for
... family of transition metal oxides, which can be used either as electrocatalysts, or in the case of semiconducting metal oxides, for integrated catalysis/photoconversion. The most efficient electrocatalysts typically employ precious metals, such as Pt, Ru and Ir. They are usually driven with an extern ...
... family of transition metal oxides, which can be used either as electrocatalysts, or in the case of semiconducting metal oxides, for integrated catalysis/photoconversion. The most efficient electrocatalysts typically employ precious metals, such as Pt, Ru and Ir. They are usually driven with an extern ...
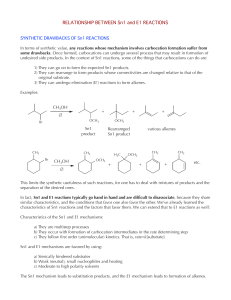
RELATIONSHIP BETWEEN Sn1 and E1 REACTIONS
... This limits the synthetic usefulness of such reactions, for one has to deal with mixtures of products and the separation of the desired ones. In fact, Sn1 and E1 reactions typically go hand in hand and are difficult to disassociate, because they share similar characteristics, and the conditions that ...
... This limits the synthetic usefulness of such reactions, for one has to deal with mixtures of products and the separation of the desired ones. In fact, Sn1 and E1 reactions typically go hand in hand and are difficult to disassociate, because they share similar characteristics, and the conditions that ...
GRADE 11F: Chemistry 6
... Use the example of the substitution of halogenoalkanes by ammonia to identify the functional group amine. In a teacher-led session, outline the main rules for naming amines. Arrange students into pairs and give them a list of names of a variety of amines. Ask them to build models of them using ‘ball ...
... Use the example of the substitution of halogenoalkanes by ammonia to identify the functional group amine. In a teacher-led session, outline the main rules for naming amines. Arrange students into pairs and give them a list of names of a variety of amines. Ask them to build models of them using ‘ball ...
reactions of alcohols with alkenes over an aluminum
... Figure 1. Mechanism for the acid-catalyzed reaction of 2-methyl pent-2-ene with alcohols (Al-montmorillonite catalyst). methyl t-butyl ether when using a clay catalyst (Bylina et al.. 1980: Adams et al.. 1981b). At this temperature methanol is the only alcohol to form a di-alkyl ether. whereas Balla ...
... Figure 1. Mechanism for the acid-catalyzed reaction of 2-methyl pent-2-ene with alcohols (Al-montmorillonite catalyst). methyl t-butyl ether when using a clay catalyst (Bylina et al.. 1980: Adams et al.. 1981b). At this temperature methanol is the only alcohol to form a di-alkyl ether. whereas Balla ...
Stereocenter www.AssignmentPoint.com A stereocenter or
... atoms) in a spatial arrangement which is not superposable on its mirror image. A chiral center is a generalized extension of an asymmetric carbon atom, which is a carbon atom bonded to four different entities, such that an interchanging of any two groups gives rise to an enantiomer. In organic chemi ...
... atoms) in a spatial arrangement which is not superposable on its mirror image. A chiral center is a generalized extension of an asymmetric carbon atom, which is a carbon atom bonded to four different entities, such that an interchanging of any two groups gives rise to an enantiomer. In organic chemi ...
Hydroformylation

Hydroformylation, also known as oxo synthesis or oxo process, is an important homogeneously catalyzed industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention in 1938: Production capacity reached 6.6×106 tons in 1995. It is important because the resulting aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to plasticizers or detergents. Hydroformylation is also used in specialty chemicals, relevant to the organic synthesis of fragrances and natural products. The development of hydroformylation, which originated within the German coal-based industry, is considered one of the premier achievements of 20th-century industrial chemistry.The process typically entails treatment of an alkene with high pressures (between 10 to 100 atmospheres) of carbon monoxide and hydrogen at temperatures between 40 and 200 °C. Transition metal catalysts are required.