Unit_4_Notes_
... collisions which leads to a faster the reaction rate. For gases, this means that the pressure and reaction rate are directly related. o Temperature at which the reaction occurs: reaction rate and temperature are directly related. An increase in temperature means an increase in kinetic energy of mole ...
... collisions which leads to a faster the reaction rate. For gases, this means that the pressure and reaction rate are directly related. o Temperature at which the reaction occurs: reaction rate and temperature are directly related. An increase in temperature means an increase in kinetic energy of mole ...
Cambridge International Examinations Cambridge
... (b) When concentrated aqueous sodium chloride is electrolysed, chlorine is formed at the positive electrode (anode) and hydrogen at the negative electrode (cathode). (i) ...
... (b) When concentrated aqueous sodium chloride is electrolysed, chlorine is formed at the positive electrode (anode) and hydrogen at the negative electrode (cathode). (i) ...
1. Naturally occurring boron consists of two isotopes, boron–10 and
... increased by a factor of 363/323. C) The average kinetic energy of the sample increased by a factor of 9/5. D) The average kinetic energy increased by a factor of 81/25. E) More information is needed to know whether the ...
... increased by a factor of 363/323. C) The average kinetic energy of the sample increased by a factor of 9/5. D) The average kinetic energy increased by a factor of 81/25. E) More information is needed to know whether the ...
Name: Date: Page 1 of 3 Organic Alcohols You are researching the
... You are researching the possibility of life forms on a planet orbiting a neighboring twin star. In the chemical spectra from the system, you discover organic alcohols that would point to the presence of oxygen and perhaps living organisms. Molecules of specific organic alcohols are pictured below. T ...
... You are researching the possibility of life forms on a planet orbiting a neighboring twin star. In the chemical spectra from the system, you discover organic alcohols that would point to the presence of oxygen and perhaps living organisms. Molecules of specific organic alcohols are pictured below. T ...
Rational design of single-site heterogeneous catalysts: towards high
... starting from the 1990s, owing to the development of silica-based mesoporous molecular sieves, larger reactant molecules, which can enter the pore network, react there and leave it, can be transformed within the inner space of porous solids (Xiao 2005; Yang et al. 2009). Mesoporous silica materials, ...
... starting from the 1990s, owing to the development of silica-based mesoporous molecular sieves, larger reactant molecules, which can enter the pore network, react there and leave it, can be transformed within the inner space of porous solids (Xiao 2005; Yang et al. 2009). Mesoporous silica materials, ...
04_01_03.html
... Overview of Chapter This chapter introduces chemical reactions and their mechanisms by focusing on two reactions that yield alkyl halides. (1) alcohol + hydrogen halide ROH + HX RX + H2O (2) alkane + halogen RH + X2 RX + HX Both are substitution reactions ...
... Overview of Chapter This chapter introduces chemical reactions and their mechanisms by focusing on two reactions that yield alkyl halides. (1) alcohol + hydrogen halide ROH + HX RX + H2O (2) alkane + halogen RH + X2 RX + HX Both are substitution reactions ...
Bricks and Shapes - Graphite Cova GmbH
... CECOLIT brickwork is thus exceptionally resistant to the mechanical demands made e.g. in numerous chemical processes due to the presence of erosive solid materials. An important characteristic of CECOLIT is its excellent stability to temperature changes which results from the good heat conductivity, ...
... CECOLIT brickwork is thus exceptionally resistant to the mechanical demands made e.g. in numerous chemical processes due to the presence of erosive solid materials. An important characteristic of CECOLIT is its excellent stability to temperature changes which results from the good heat conductivity, ...
printable - Master Organic Chemistry
... KMnO4, acid, heat cleaves C=C to give two carbonyls. Alkenyl C-H bonds oxidized to C–OH ...
... KMnO4, acid, heat cleaves C=C to give two carbonyls. Alkenyl C-H bonds oxidized to C–OH ...
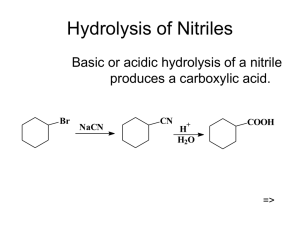
Hydrolysis of Nitriles
... Chloride is a good leaving group, so undergoes acyl substitution easily. To synthesize acid chlorides use thionyl chloride or oxalyl chloride with the acid. O ...
... Chloride is a good leaving group, so undergoes acyl substitution easily. To synthesize acid chlorides use thionyl chloride or oxalyl chloride with the acid. O ...
12602989_294 - University of Canterbury
... furnished two products, (2) and (3), each of which had 1:1 metal:ligand stoichiometry. X-Ray crystal structure determination9 revealed that complex 2 is an M2L2 metallomacrocycle, in which two silver atoms are bridged by two para-divinylbenzene ligands, with η2-coordination by the vinyl groups and a ...
... furnished two products, (2) and (3), each of which had 1:1 metal:ligand stoichiometry. X-Ray crystal structure determination9 revealed that complex 2 is an M2L2 metallomacrocycle, in which two silver atoms are bridged by two para-divinylbenzene ligands, with η2-coordination by the vinyl groups and a ...
Presentation
... 2. What is a ligand? Give examples. g is defined as an ion, atom A ligand or a molecule capable of donating one or more pairs of electrons to the central metal ion or atom. ...
... 2. What is a ligand? Give examples. g is defined as an ion, atom A ligand or a molecule capable of donating one or more pairs of electrons to the central metal ion or atom. ...
2.7 INTRODUCTION TO FUNCTIONAL GROUPS
... have similar physical properties. The polarity and hydrogen bonding of the OH group cause both of them to have higher boiling points than similar compounds containing only C and H. Because the second one has a greater molecular mass, it is expected to have a somewhat higher boiling point. The OH gro ...
... have similar physical properties. The polarity and hydrogen bonding of the OH group cause both of them to have higher boiling points than similar compounds containing only C and H. Because the second one has a greater molecular mass, it is expected to have a somewhat higher boiling point. The OH gro ...
Chemistry in Society - Cathkin High School
... Crude oil is a raw material from which naphtha is obtained by fractional distillation. Naphtha is a feedstock that can be cracked to produce ethene. Batch and Continuous Processes In a batch process the chemicals are loaded into the reaction vessel. The reaction is monitored and at the end of the re ...
... Crude oil is a raw material from which naphtha is obtained by fractional distillation. Naphtha is a feedstock that can be cracked to produce ethene. Batch and Continuous Processes In a batch process the chemicals are loaded into the reaction vessel. The reaction is monitored and at the end of the re ...
Chapter 3 Ligand Effects
... 3.1.1 Studies of ligand effects on Lewis-acid catalysed reactions in water Research on ligand effects in aqueous solution has mainly focused on two types of organic reactions: decarboxylation and hydrolysis reactions. In section 2.1.1 the Lewis-acid catalysis of the decarboxylation of oxaloacetate w ...
... 3.1.1 Studies of ligand effects on Lewis-acid catalysed reactions in water Research on ligand effects in aqueous solution has mainly focused on two types of organic reactions: decarboxylation and hydrolysis reactions. In section 2.1.1 the Lewis-acid catalysis of the decarboxylation of oxaloacetate w ...
Ester - SCH4U-SRB
... Esters are responsible for the “fruity” odours and flavours of many naturally occurring products. Chemists can reproduce these odours or flavours in the lab by mixing the right alcohol and carboxylic acid together; thus, producing “artificial” or “synthetic” versions of these naturally occurring com ...
... Esters are responsible for the “fruity” odours and flavours of many naturally occurring products. Chemists can reproduce these odours or flavours in the lab by mixing the right alcohol and carboxylic acid together; thus, producing “artificial” or “synthetic” versions of these naturally occurring com ...
Hydrocarbon Derivatives:
... • contain only carbon & hydrogen • carbon can also form strong covalent bonds with other elements such as: O, N, F, Cl, Br, I, S, & P ...
... • contain only carbon & hydrogen • carbon can also form strong covalent bonds with other elements such as: O, N, F, Cl, Br, I, S, & P ...
Lab 9
... Esters are acid derivatives and contain a carbonyl group; whereas, ethers are water or alcohol derivatives and do not contain a carbonyl group. Alcohols are named by finding the longest carbon chain to which the OH group is bonded and naming the alcohol accordingly. Ethers have two R groups, which m ...
... Esters are acid derivatives and contain a carbonyl group; whereas, ethers are water or alcohol derivatives and do not contain a carbonyl group. Alcohols are named by finding the longest carbon chain to which the OH group is bonded and naming the alcohol accordingly. Ethers have two R groups, which m ...
Hydroformylation

Hydroformylation, also known as oxo synthesis or oxo process, is an important homogeneously catalyzed industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention in 1938: Production capacity reached 6.6×106 tons in 1995. It is important because the resulting aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to plasticizers or detergents. Hydroformylation is also used in specialty chemicals, relevant to the organic synthesis of fragrances and natural products. The development of hydroformylation, which originated within the German coal-based industry, is considered one of the premier achievements of 20th-century industrial chemistry.The process typically entails treatment of an alkene with high pressures (between 10 to 100 atmospheres) of carbon monoxide and hydrogen at temperatures between 40 and 200 °C. Transition metal catalysts are required.