Ch-6-Alcohols and phenols - Home
... Physical Properties of Phenols • Phenol is a crystalline solid, with a higher boiling point than alcohols, and it is moderately soluble in water. • It is slightly acidic: the phenol molecule has weak tendencies to lose the H+ ion from the hydroxyl group, resulting in the highly water-soluble phenol ...
... Physical Properties of Phenols • Phenol is a crystalline solid, with a higher boiling point than alcohols, and it is moderately soluble in water. • It is slightly acidic: the phenol molecule has weak tendencies to lose the H+ ion from the hydroxyl group, resulting in the highly water-soluble phenol ...
amine
... ◦ When four atoms or groups of atoms are bonded to a nitrogen atom, as for example CH3NH3+, nitrogen bears a positive charge and is associated with an anion as a salt. ◦ Name the compound as a salt of the corresponding amine. ◦ Replace the ending –amine (or aniline or pyridine or the like) by -ammon ...
... ◦ When four atoms or groups of atoms are bonded to a nitrogen atom, as for example CH3NH3+, nitrogen bears a positive charge and is associated with an anion as a salt. ◦ Name the compound as a salt of the corresponding amine. ◦ Replace the ending –amine (or aniline or pyridine or the like) by -ammon ...
Aromatic Compounds
... • Addition of a reagent such as HCl to an alkene • The electrophilic hydrogen approaches the p electrons ...
... • Addition of a reagent such as HCl to an alkene • The electrophilic hydrogen approaches the p electrons ...
Factors influencing ring closure through olefin metathesis – A
... available, cyclization is the most straightforward way of obtaining rings. Commonly employed cyclization methods involve reactions of cationic, anionic or radical species. Common rings such as 5–7 membered ones are easily available by these methods. However, formation of medium or large rings by the ...
... available, cyclization is the most straightforward way of obtaining rings. Commonly employed cyclization methods involve reactions of cationic, anionic or radical species. Common rings such as 5–7 membered ones are easily available by these methods. However, formation of medium or large rings by the ...
lecture 6 oxidative addition
... however, a vacant 2e site is always required on the metal. • We can either start with a 16e complex or a 2e site must be opened up in an 18e complex by the loss of a ligand producing a 16e intermediate species. • The change in oxidation state means that the starting metal complex of a given oxidatio ...
... however, a vacant 2e site is always required on the metal. • We can either start with a 16e complex or a 2e site must be opened up in an 18e complex by the loss of a ligand producing a 16e intermediate species. • The change in oxidation state means that the starting metal complex of a given oxidatio ...
Reactions of carbon radicals generated by 1,5
... sors of alkoxyl radicals, and can be prepared in a separate reaction and then subjected to photolytic decomposition.14 Among the most convenient and easily available precursors of alkoxyl radicals are the alkyl nitrites 4b (RO-NO, 222, kJ/mol), prepared by the esterification of alcohols with nitrous ...
... sors of alkoxyl radicals, and can be prepared in a separate reaction and then subjected to photolytic decomposition.14 Among the most convenient and easily available precursors of alkoxyl radicals are the alkyl nitrites 4b (RO-NO, 222, kJ/mol), prepared by the esterification of alcohols with nitrous ...
SYNTHESIS OF NEW DICLOFENAC DERIVATIVES BY COUPLING WITH CHALCONE
... Chalcone [1, 3-diphenyl-2-propene-1-one] and related compounds "chalconoids" are those in which two aromatic rings are linked and conjugated together through a reactive keto ethylenic linkage (–CO– CH=CH–), which impart an entirely delocalized π-electron system on both benzene rings in such a way th ...
... Chalcone [1, 3-diphenyl-2-propene-1-one] and related compounds "chalconoids" are those in which two aromatic rings are linked and conjugated together through a reactive keto ethylenic linkage (–CO– CH=CH–), which impart an entirely delocalized π-electron system on both benzene rings in such a way th ...
isomeria geometrica
... Stereocenters • Any atom at which the exchange of two groups yields a stereoisomer. • Examples: • Asymmetric carbons • Double-bonded carbons in cis-trans isomers ...
... Stereocenters • Any atom at which the exchange of two groups yields a stereoisomer. • Examples: • Asymmetric carbons • Double-bonded carbons in cis-trans isomers ...
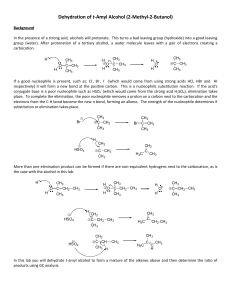
Dehydration of t-Amyl Alcohol (2-Methyl-2
... If a good nucleophile is present, such as: Cl-, Br-, I- (which would come from using strong acids HCl, HBr and HI respectively) it will form a new bond at the positive carbon. This is a nucleophilic substitution reaction. If the acid's conjugate base is a poor nucleophile such as HSO4- (which would ...
... If a good nucleophile is present, such as: Cl-, Br-, I- (which would come from using strong acids HCl, HBr and HI respectively) it will form a new bond at the positive carbon. This is a nucleophilic substitution reaction. If the acid's conjugate base is a poor nucleophile such as HSO4- (which would ...
CBSE Class 12 Chemistry notes and questions for Alcohols Phenols
... characteristic colour with aqueous FeCl . When A is treated with NaOH upressure, compound B is obtained. Compound t and CO at 400 K under s . compound C which reacts with acetyl chloride to B on acidification gives w form D which isw a popular pain killer. Deduce the structure of A, B, C and w D. Wh ...
... characteristic colour with aqueous FeCl . When A is treated with NaOH upressure, compound B is obtained. Compound t and CO at 400 K under s . compound C which reacts with acetyl chloride to B on acidification gives w form D which isw a popular pain killer. Deduce the structure of A, B, C and w D. Wh ...
Alcohols, Phenols, and Ethers
... c) isopropyl alcohol and propylene glycol d) t-butyl alcohol and s-butyl alcohol ...
... c) isopropyl alcohol and propylene glycol d) t-butyl alcohol and s-butyl alcohol ...
chemistry - Canisteo-Greenwood Central School
... • Denatured alcohol is ethanol with an added substance to make it toxic (poisonous). ...
... • Denatured alcohol is ethanol with an added substance to make it toxic (poisonous). ...
Alcohols and Ethers
... • Denatured alcohol is ethanol with an added substance to make it toxic (poisonous). ...
... • Denatured alcohol is ethanol with an added substance to make it toxic (poisonous). ...
End Show - Lisle CUSD 202
... Aliphatic alcohols can be classified into structural categories according to the number of R groups attached to the carbon with the hydroxyl group. ...
... Aliphatic alcohols can be classified into structural categories according to the number of R groups attached to the carbon with the hydroxyl group. ...
Organic Synthesis II
... 1. Oxidation & Reduction in Organic Synthesis (T. J. Donohoe, OUP) 2. Organic Chemistry (Clayden, Greene, Wothers & Warren, OUP) ...
... 1. Oxidation & Reduction in Organic Synthesis (T. J. Donohoe, OUP) 2. Organic Chemistry (Clayden, Greene, Wothers & Warren, OUP) ...
File
... strong signal at 1691 cm-1. Both signals represent vibration of the same kind of bond. Explain why the absorption in 2-cyclohexenone is at a lower wavenumber, including resonance structures. Both signals represent the IR stretching of the C=O bonds. 2-cyclohexenone has a lower wavenumber absorbance, ...
... strong signal at 1691 cm-1. Both signals represent vibration of the same kind of bond. Explain why the absorption in 2-cyclohexenone is at a lower wavenumber, including resonance structures. Both signals represent the IR stretching of the C=O bonds. 2-cyclohexenone has a lower wavenumber absorbance, ...
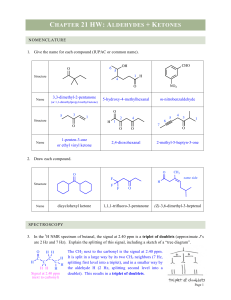
4797 Chem Test Ch 21
... chlorine atoms. The released chlorine reacts with ozone to form ClO and O2. The ClO combines with atomic oxygen to produce more chlorine atoms. These then react with more ozone molecules. Ozone molecules are consumed in these reactions. 35. In aldehydes, the carbonyl group is attached to a carbon at ...
... chlorine atoms. The released chlorine reacts with ozone to form ClO and O2. The ClO combines with atomic oxygen to produce more chlorine atoms. These then react with more ozone molecules. Ozone molecules are consumed in these reactions. 35. In aldehydes, the carbonyl group is attached to a carbon at ...
Aldehydes Ketones Carboxylic Acid
... (ii) Ketones are generally oxidized under drastic conditions i.e. with powerful oxidising agents like conc. HNO3, KMnO4/ H2SO4 , K2Cr2O7/ H2SO4 at elevated temperature ...
... (ii) Ketones are generally oxidized under drastic conditions i.e. with powerful oxidising agents like conc. HNO3, KMnO4/ H2SO4 , K2Cr2O7/ H2SO4 at elevated temperature ...
Slide 1
... • Thiols have lower boiling points and are less soluble in water and other polar solvents than alcohols of similar molecular weight. ...
... • Thiols have lower boiling points and are less soluble in water and other polar solvents than alcohols of similar molecular weight. ...
CHM 103 Lecture 24 S07
... • are named in the IUPAC system by adding thiol to the alkane name of the longest carbon chain. • the -SH group may also be called a “mercapto” group ...
... • are named in the IUPAC system by adding thiol to the alkane name of the longest carbon chain. • the -SH group may also be called a “mercapto” group ...
Organic Chemistry Fifth Edition
... Mechanisms are often written as a series of chemical equations showing the elementary steps. An elementary step is a reaction that proceeds by way of a single transition state. Mechanisms can be shown likely to be correct, but cannot be proven correct. ...
... Mechanisms are often written as a series of chemical equations showing the elementary steps. An elementary step is a reaction that proceeds by way of a single transition state. Mechanisms can be shown likely to be correct, but cannot be proven correct. ...
BIOO211 SN05 Lecture OrganicChem
... Learning Check, Naming Example The structural formula is drawn from the IUPAC name. 2, 3-dimethylpentane ...
... Learning Check, Naming Example The structural formula is drawn from the IUPAC name. 2, 3-dimethylpentane ...
Process for preparing polycarbonates
... and cleansing. Furthermore, in many of these industrial and household applications it is necessary to maintain a ...
... and cleansing. Furthermore, in many of these industrial and household applications it is necessary to maintain a ...
Alkene

In organic chemistry, an alkene is an unsaturated hydrocarbon that contains at least one carbon–carbon double bond. Alkene, olefin, and olefine are used often interchangeably (see nomenclature section below). Acyclic alkenes, with only one double bond and no other functional groups, known as mono-enes, form a homologous series of hydrocarbons with the general formula CnH2n. Alkenes have two hydrogen atoms less than the corresponding alkane (with the same number of carbon atoms). The simplest alkene, ethylene (C2H4), which has the International Union of Pure and Applied Chemistry (IUPAC) name ethene is the organic compound produced on the largest scale industrially. Aromatic compounds are often drawn as cyclic alkenes, but their structure and properties are different and they are not considered to be alkenes.