PROFESSOR SIR DEREK H. R. BARTON AND HETEROCYCLES
... saponification furnished the 20-oxopregnane derivative in good yield. ...
... saponification furnished the 20-oxopregnane derivative in good yield. ...
Polymerization Lab
... 2) Initiation: Using an oxygen atom, in this case, representing a free radical, initiate the polymerization by breaking one of the double bonds and connecting it to the oxygen free radical. a. Remember that a free radical is an atom that has an unpaired electron. So, of the two electrons in the bond ...
... 2) Initiation: Using an oxygen atom, in this case, representing a free radical, initiate the polymerization by breaking one of the double bonds and connecting it to the oxygen free radical. a. Remember that a free radical is an atom that has an unpaired electron. So, of the two electrons in the bond ...
Topic 22 Notes
... Organic chemistry is important because there are more compounds formed from carbon than all of the other elements put together. B. Organic molecules have covalent bonds. 1. Non-polar and polar bonds a. Non-polar bonds. There is equal or nearly equal sharing of the bonding electrons between the two a ...
... Organic chemistry is important because there are more compounds formed from carbon than all of the other elements put together. B. Organic molecules have covalent bonds. 1. Non-polar and polar bonds a. Non-polar bonds. There is equal or nearly equal sharing of the bonding electrons between the two a ...
Reactions of Alcohols
... • Add ZnCl2, which bonds strongly with -OH, to promote the reaction. • The chloride product is insoluble. • Lucas test: ZnCl2 in conc. HCl 1° alcohols react slowly or not at all. 2 alcohols react in 1-5 minutes. 3 alcohols react in less than 1 minute. ...
... • Add ZnCl2, which bonds strongly with -OH, to promote the reaction. • The chloride product is insoluble. • Lucas test: ZnCl2 in conc. HCl 1° alcohols react slowly or not at all. 2 alcohols react in 1-5 minutes. 3 alcohols react in less than 1 minute. ...
PHYSICOCHEMICAL PROPERTIES OF ORGANIC MEDICINAL
... CH3(CH2)4-OH (1-Pentanol) As a result of intermolecular H-bonding, alcohols have significantly higher boiling points than corresponding simple hydrocarbons. For example, simple hydrocarbons with fewer than five ...
... CH3(CH2)4-OH (1-Pentanol) As a result of intermolecular H-bonding, alcohols have significantly higher boiling points than corresponding simple hydrocarbons. For example, simple hydrocarbons with fewer than five ...
Alcools
... The OH group has a higher priority than a multiple CC bond, a halogen, and an alkyl group in determining the carbon chain numbering. ...
... The OH group has a higher priority than a multiple CC bond, a halogen, and an alkyl group in determining the carbon chain numbering. ...
Chem 322 - Exam #3 - Spring 2003
... the structure of compound C. You would need to recall that when esters form from alcohols and acids, the acid loses an OH, the alcohol loses an H, and the ester bond forms. If you were less astute, the structure of the final product should have told you that compound C has five carbons and the 2,2-d ...
... the structure of compound C. You would need to recall that when esters form from alcohols and acids, the acid loses an OH, the alcohol loses an H, and the ester bond forms. If you were less astute, the structure of the final product should have told you that compound C has five carbons and the 2,2-d ...
Aldehydes and Ketones - Belle Vernon Area School District
... IUPAC - # chain from the end closest to the carbonyl, give the carbon #, drop –e add –one Name the groups on either side of the carbonyl and follow with ketone list groups by order of sixe of molar mass (smaller 1st) ...
... IUPAC - # chain from the end closest to the carbonyl, give the carbon #, drop –e add –one Name the groups on either side of the carbonyl and follow with ketone list groups by order of sixe of molar mass (smaller 1st) ...
Unit 13: Organic Chemistry
... Objective: What are Alkenes, and how do they function in chemistry? Alkene Family: 1. The alkene family, also known as the olefin family, differ from their related alkanes by having one carbon to carbon double bond (C=C) somewhere along the longest chain. 2. Ethane (C2H4) and propene (C3H6) are the ...
... Objective: What are Alkenes, and how do they function in chemistry? Alkene Family: 1. The alkene family, also known as the olefin family, differ from their related alkanes by having one carbon to carbon double bond (C=C) somewhere along the longest chain. 2. Ethane (C2H4) and propene (C3H6) are the ...
Number of students performing at
... occur following either an SN1 or an SN2 mechanism. Based on the structure of each of the alcohols, one can make a prediction on the mechanism of its reaction with HBr, as shown above to the right. The profile, on the other hand, definitely reflects a stepwise reaction, i.e. one that follows an SN1 m ...
... occur following either an SN1 or an SN2 mechanism. Based on the structure of each of the alcohols, one can make a prediction on the mechanism of its reaction with HBr, as shown above to the right. The profile, on the other hand, definitely reflects a stepwise reaction, i.e. one that follows an SN1 m ...
幻灯片 1
... 1846.His ability to work in laboratory was hampered by a childhood injury that caused the loss of an arm. From 1849,utill 1887, he was professor of Chemistry at University College, London. ...
... 1846.His ability to work in laboratory was hampered by a childhood injury that caused the loss of an arm. From 1849,utill 1887, he was professor of Chemistry at University College, London. ...
Unit 13: Organic Chemistry
... Objective: What are Alkenes, and how do they function in chemistry? Alkene Family: 1. The alkene family, also known as the olefin family, differ from their related alkanes by having one carbon to carbon double bond (C=C) somewhere along the longest chain. 2. Ethane (C2H4) and propene (C3H6) are the ...
... Objective: What are Alkenes, and how do they function in chemistry? Alkene Family: 1. The alkene family, also known as the olefin family, differ from their related alkanes by having one carbon to carbon double bond (C=C) somewhere along the longest chain. 2. Ethane (C2H4) and propene (C3H6) are the ...
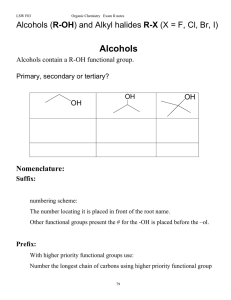
Alcohols (R-OH), and alkyl halides, RX
... Step 1: What are the possible monosubstituted products? Step 2: How many hydrogens that yield the same product? (nHi) Step 3: What is the reactivity (Ri) for the hydrogen and halogen type? Step 4: Using the reactivity factors, what are the total possible structures? Step 5: What is the percent of ea ...
... Step 1: What are the possible monosubstituted products? Step 2: How many hydrogens that yield the same product? (nHi) Step 3: What is the reactivity (Ri) for the hydrogen and halogen type? Step 4: Using the reactivity factors, what are the total possible structures? Step 5: What is the percent of ea ...
General and Selective Synthesis of (Z)-3
... oxidation reaction of Pd(0) with CuX2 to start a new catalytic cycle. In summary, PdX2 catalyzed carbonylation of terminal alkynes with CO and various alcohols including the bulky tertiary alcohol in the presence of CuX2 to form (Z)-3haloacrylates (halo ) Cl and Br) in moderate to good yields with e ...
... oxidation reaction of Pd(0) with CuX2 to start a new catalytic cycle. In summary, PdX2 catalyzed carbonylation of terminal alkynes with CO and various alcohols including the bulky tertiary alcohol in the presence of CuX2 to form (Z)-3haloacrylates (halo ) Cl and Br) in moderate to good yields with e ...
Chapter 11 Reactions of Alcohols Types of Alcohol Reactions
... • Add ZnCl2, which bonds strongly with -OH, to promote the reaction. • The chloride product is insoluble. • Lucas test: ZnCl2 in conc. HCl ¾1° alcohols react slowly or not at all. ¾2° alcohols react in 1-5 minutes. ¾3° alcohols react in less than 1 minute. ...
... • Add ZnCl2, which bonds strongly with -OH, to promote the reaction. • The chloride product is insoluble. • Lucas test: ZnCl2 in conc. HCl ¾1° alcohols react slowly or not at all. ¾2° alcohols react in 1-5 minutes. ¾3° alcohols react in less than 1 minute. ...
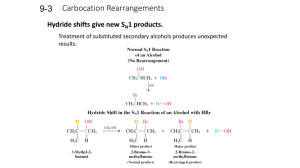
Carbocation Rearrangements
... Alkyl and hydride shifts to primary carbons bearing leaving groups can occur without the formation of primary carbocations. ...
... Alkyl and hydride shifts to primary carbons bearing leaving groups can occur without the formation of primary carbocations. ...
chemistry - Textbooks Online
... form a molecule" is required to gain knowledge of the followingi) to know about how atoms of same element form different compounds combining with different elements. ii) to know why particular shapes are adopted by molecules. iii) to understand the specific properties of molecules or ions and the re ...
... form a molecule" is required to gain knowledge of the followingi) to know about how atoms of same element form different compounds combining with different elements. ii) to know why particular shapes are adopted by molecules. iii) to understand the specific properties of molecules or ions and the re ...
7: Reactions of Haloalkanes, Alcohols, and Amines. Nucleophilic
... alcohols, amines, and compounds related to them. These are ionic reactions in which one group on the molecule (a leaving group) is replaced by another group (a nucleophile). The transformation of haloalkanes (R-X) into alcohols (R-OH) where an OH group replaces the halogen (X) is an example of nucle ...
... alcohols, amines, and compounds related to them. These are ionic reactions in which one group on the molecule (a leaving group) is replaced by another group (a nucleophile). The transformation of haloalkanes (R-X) into alcohols (R-OH) where an OH group replaces the halogen (X) is an example of nucle ...
Alkene

In organic chemistry, an alkene is an unsaturated hydrocarbon that contains at least one carbon–carbon double bond. Alkene, olefin, and olefine are used often interchangeably (see nomenclature section below). Acyclic alkenes, with only one double bond and no other functional groups, known as mono-enes, form a homologous series of hydrocarbons with the general formula CnH2n. Alkenes have two hydrogen atoms less than the corresponding alkane (with the same number of carbon atoms). The simplest alkene, ethylene (C2H4), which has the International Union of Pure and Applied Chemistry (IUPAC) name ethene is the organic compound produced on the largest scale industrially. Aromatic compounds are often drawn as cyclic alkenes, but their structure and properties are different and they are not considered to be alkenes.