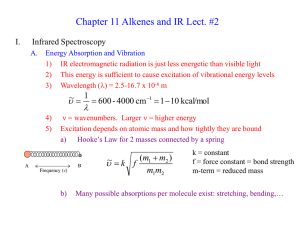
Unit 13: Organic Chemistry
... Objective: What are Alkenes, and how do they function in chemistry? Alkene Family: 1. The alkene family, also known as the olefin family, differ from their related alkanes by having one carbon to carbon double bond (C=C) somewhere along the longest chain. 2. Ethane (C2H4) and propene (C3H6) are the ...
... Objective: What are Alkenes, and how do they function in chemistry? Alkene Family: 1. The alkene family, also known as the olefin family, differ from their related alkanes by having one carbon to carbon double bond (C=C) somewhere along the longest chain. 2. Ethane (C2H4) and propene (C3H6) are the ...
Lecture - Ch 19
... intermediate, which expels hydride ion as a leaving group and is thereby oxidized – A second aldehyde molecule accepts the hydride ion in another nucleophilic addition step and is thereby reduced ...
... intermediate, which expels hydride ion as a leaving group and is thereby oxidized – A second aldehyde molecule accepts the hydride ion in another nucleophilic addition step and is thereby reduced ...
PREPARATION, STRUCTURAL STUDIES AND CHEMICAL
... 1. The first reported preparation of a hypervalent iodine (III) compound…………..……1 2. Structural types of hypervalent iodine compounds…………………………………….2 3. Common classes of hypervalent iodine(III) and (V) compounds…………………….. 4 4. Known classes of heterocyclic hypervalent iodine compounds………………..………5 5 ...
... 1. The first reported preparation of a hypervalent iodine (III) compound…………..……1 2. Structural types of hypervalent iodine compounds…………………………………….2 3. Common classes of hypervalent iodine(III) and (V) compounds…………………….. 4 4. Known classes of heterocyclic hypervalent iodine compounds………………..………5 5 ...
Chapter 1 Structure and Bonding
... Hyperconjugation makes substituted alkenes more stable by stabilizing p-orbitals cis alkenes are less stable than trans alkenes because of steric crowding cis cycloalkenes are more stable than trans for the small rings ...
... Hyperconjugation makes substituted alkenes more stable by stabilizing p-orbitals cis alkenes are less stable than trans alkenes because of steric crowding cis cycloalkenes are more stable than trans for the small rings ...
lecture 7 reductive eliminations
... • Agostic complexes, σ complexes of C−H bonds, can be thought of as lying along the pathway for oxidative addition but arrested at different points. ...
... • Agostic complexes, σ complexes of C−H bonds, can be thought of as lying along the pathway for oxidative addition but arrested at different points. ...
Process for preparing polycarbonates
... alkali metal salt catalyst are sodium stannate, potassium The reaction conditions of Examples 1 and 2 were stannate, sodium carbonate, potassium carbonate, so dium hydroxide, potassium hydroxide, the sodium alk 25 duplicated except that a mixture of 44 grams (0.5 moles) oxide of the monohydroxy alco ...
... alkali metal salt catalyst are sodium stannate, potassium The reaction conditions of Examples 1 and 2 were stannate, sodium carbonate, potassium carbonate, so dium hydroxide, potassium hydroxide, the sodium alk 25 duplicated except that a mixture of 44 grams (0.5 moles) oxide of the monohydroxy alco ...
Oxidation catalytic system and oxidation process using the same
... Incidentally, the compounds, Which are substituted With a hydroxyl group on a tertiary carbon atom of a junction site ...
... Incidentally, the compounds, Which are substituted With a hydroxyl group on a tertiary carbon atom of a junction site ...
Some uses of mischmetall in organic synthesis
... regeneration of SmI2 (or SmX2). In the catalytic scheme II, the initially formed RSmIX reacts with Ln to give another organometallic compound (LnR3; Ln ≠ Sm) through a reduction-transmetallation process, with the regeneration of SmI2. This organolanthanoid compound then adds to the carbonyl compound ...
... regeneration of SmI2 (or SmX2). In the catalytic scheme II, the initially formed RSmIX reacts with Ln to give another organometallic compound (LnR3; Ln ≠ Sm) through a reduction-transmetallation process, with the regeneration of SmI2. This organolanthanoid compound then adds to the carbonyl compound ...
Forward
... As we’ll see later in this chapter and the next, aldehydes and ketones are involved in many of the most used reactions in synthetic organic chemistry. Where do aldehydes and ketones come from? Many occur naturally. In terms of both variety and quantity, aldehydes and ketones rank among the most comm ...
... As we’ll see later in this chapter and the next, aldehydes and ketones are involved in many of the most used reactions in synthetic organic chemistry. Where do aldehydes and ketones come from? Many occur naturally. In terms of both variety and quantity, aldehydes and ketones rank among the most comm ...
Chapter 25 Organic and Biological Chemistry
... • All of this is of course now on the web. I’ll put the chap 5 notes on the web as well today, though they are not “perfected” yet. ...
... • All of this is of course now on the web. I’ll put the chap 5 notes on the web as well today, though they are not “perfected” yet. ...
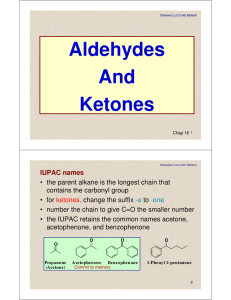
Aldehydes And Ketones
... • Use double-barbed arrows to indicate the flow of pairs of e• Draw the arrow from higher e- density to lower e- density i.e. from the nucleophile to the electrophile • Removing e- density from an atom will create a formal + charge • Adding e- density to an atom will create a formal - charge • Proto ...
... • Use double-barbed arrows to indicate the flow of pairs of e• Draw the arrow from higher e- density to lower e- density i.e. from the nucleophile to the electrophile • Removing e- density from an atom will create a formal + charge • Adding e- density to an atom will create a formal - charge • Proto ...
BIOO211 SN05 Lecture OrganicChem
... Many carbon branches use their alkyl group names. Timberlake 2016, p.485 © 2016 Pearson Education, Inc. ...
... Many carbon branches use their alkyl group names. Timberlake 2016, p.485 © 2016 Pearson Education, Inc. ...
BSA - Sigma
... glycol phases). Silicones are the most useful phases for TMS derivatives – they combine inertness and stability with excellent separating characteristics for these derivatives. Nonpolar silicone phases include SPB™-1 and SPB-5. Normal hydrocarbons (carbonhydrogen analytes with single bonds) are sepa ...
... glycol phases). Silicones are the most useful phases for TMS derivatives – they combine inertness and stability with excellent separating characteristics for these derivatives. Nonpolar silicone phases include SPB™-1 and SPB-5. Normal hydrocarbons (carbonhydrogen analytes with single bonds) are sepa ...
review-rough
... 21. A substitute for silk, this substance is formed from the reaction of adipic acid with 1,6 diaminohexane. ...
... 21. A substitute for silk, this substance is formed from the reaction of adipic acid with 1,6 diaminohexane. ...
PHENOL - Gneet's
... The above reaction is laboratory method for preparation of phenol 2. Hydrolysis of diazonium salt ...
... The above reaction is laboratory method for preparation of phenol 2. Hydrolysis of diazonium salt ...
No Slide Title
... the carbon atoms are in a single row. The other is a branched molecule where three carbon atoms are in a row and one carbon atom sticks out of the main chain. ...
... the carbon atoms are in a single row. The other is a branched molecule where three carbon atoms are in a row and one carbon atom sticks out of the main chain. ...
Isomerism - Knockhardy
... the carbon atoms are in a single row. The other is a branched molecule where three carbon atoms are in a row and one carbon atom sticks out of the main chain. ...
... the carbon atoms are in a single row. The other is a branched molecule where three carbon atoms are in a row and one carbon atom sticks out of the main chain. ...
- Opus: Online Publications Store
... Additional problems associated with the activation of C–H bonds are their low dipole moments, low HOMO and high LUMO energy levels [9]. Furthermore, the activation methods developed should be able to introduce different functionalities under the same reaction conditions [1c,10]. Because of the previ ...
... Additional problems associated with the activation of C–H bonds are their low dipole moments, low HOMO and high LUMO energy levels [9]. Furthermore, the activation methods developed should be able to introduce different functionalities under the same reaction conditions [1c,10]. Because of the previ ...
Document
... lower than those of alcohols. Formaldehyde is a gas at room temperature (b.p. = -21 C) but heavier aldehydes are liquids. Acetone, the simplest ketone, is a liquid at room temperature (b.p. = 56 C). Lower molecular weight aldehydes and ketones are water soluble. Acetone, formaldehyde and acetaldeh ...
... lower than those of alcohols. Formaldehyde is a gas at room temperature (b.p. = -21 C) but heavier aldehydes are liquids. Acetone, the simplest ketone, is a liquid at room temperature (b.p. = 56 C). Lower molecular weight aldehydes and ketones are water soluble. Acetone, formaldehyde and acetaldeh ...
CBSE Class 12 Chemistry notes and questions for Alcohols Phenols
... characteristic colour with aqueous FeCl . When A is treated with NaOH upressure, compound B is obtained. Compound t and CO at 400 K under s . compound C which reacts with acetyl chloride to B on acidification gives w form D which isw a popular pain killer. Deduce the structure of A, B, C and w D. Wh ...
... characteristic colour with aqueous FeCl . When A is treated with NaOH upressure, compound B is obtained. Compound t and CO at 400 K under s . compound C which reacts with acetyl chloride to B on acidification gives w form D which isw a popular pain killer. Deduce the structure of A, B, C and w D. Wh ...
Carboxylic Acid Derivatives and Nucleophilic Acyl Substitution
... groups More electrophilic carbonyl groups are more reactive to addition (acyl halides are most reactive, amides are least) The intermediate with the best leaving group decomposes fastest Based on McMurry, Organic Chemistry, Chapter 21, 6th edition, (c) 2003 ...
... groups More electrophilic carbonyl groups are more reactive to addition (acyl halides are most reactive, amides are least) The intermediate with the best leaving group decomposes fastest Based on McMurry, Organic Chemistry, Chapter 21, 6th edition, (c) 2003 ...
applied sciences Chiral β-Amino Alcohols as Ligands for the N
... prochiral ketones affords enantiomerically enriched secondary alcohols with excellent results [12–19]. Nevertheless, the asymmetric reduction of iminic compounds by transfer hydrogenation yielding chiral amines still remains underdeveloped and represents a challenging objective [20,21]. Due to the l ...
... prochiral ketones affords enantiomerically enriched secondary alcohols with excellent results [12–19]. Nevertheless, the asymmetric reduction of iminic compounds by transfer hydrogenation yielding chiral amines still remains underdeveloped and represents a challenging objective [20,21]. Due to the l ...
ppt file
... groups More electrophilic carbonyl groups are more reactive to addition (acyl halides are most reactive, amides are least) The intermediate with the best leaving group decomposes fastest Based on McMurry, Organic Chemistry, Chapter 21, 6th edition, (c) 2003 ...
... groups More electrophilic carbonyl groups are more reactive to addition (acyl halides are most reactive, amides are least) The intermediate with the best leaving group decomposes fastest Based on McMurry, Organic Chemistry, Chapter 21, 6th edition, (c) 2003 ...
Reprinted from The Journal of Physical Chemistry, lgg5, 99
... molecular orbital (LUMO) of the adsorbed molecules. Both experimental and theoretical work suggeststhat the STM images at thesebias voltages do not corresponddirectly to the electronic structure of the adsorbed molecules but rather reflect perturbations of the solid substrateelectronic statesdue to ...
... molecular orbital (LUMO) of the adsorbed molecules. Both experimental and theoretical work suggeststhat the STM images at thesebias voltages do not corresponddirectly to the electronic structure of the adsorbed molecules but rather reflect perturbations of the solid substrateelectronic statesdue to ...
McMurray-Fay Chapter 22 Presentation Slides
... compounds. • Carbon is tetravalent. It has four outer-shell electrons (1s22s22p2) and forms four bonds. ...
... compounds. • Carbon is tetravalent. It has four outer-shell electrons (1s22s22p2) and forms four bonds. ...
Haloalkane

The haloalkanes (also known, as halogenoalkanes or alkyl halides) are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula ″RX″ where R is an alkyl or substituted alkyl group and X is a halogen (F, Cl, Br, I).Haloalkanes have been known for centuries. Chloroethane was produced synthetically in the 15th century. The systematic synthesis of such compounds developed in the 19th century in step with the development of organic chemistry and the understanding of the structure of alkanes. Methods were developed for the selective formation of C-halogen bonds. Especially versatile methods included the addition of halogens to alkenes, hydrohalogenation of alkenes, and the conversion of alcohols to alkyl halides. These methods are so reliable and so easily implemented that haloalkanes became cheaply available for use in industrial chemistry because the halide could be further replaced by other functional groups.While most haloalkanes are human-produced, non-artificial-source haloalkanes do occur on Earth, mostly through enzyme-mediated synthesis by bacteria, fungi, and especially sea macroalgae (seaweeds). More than 1600 halogenated organics have been identified, with bromoalkanes being the most common haloalkanes. Brominated organics in biology range from biologically produced methyl bromide to non-alkane aromatics and unsaturates (indoles, terpenes, acetogenins, and phenols). Halogenated alkanes in land plants are more rare, but do occur, as for example the fluoroacetate produced as a toxin by at least 40 species of known plants. Specific dehalogenase enzymes in bacteria which remove halogens from haloalkanes, are also known.